You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The COVID-19 pandemic has created a new way of viewing public health from an oral health perspective. While several infectious disease outbreaks have occurred in the past 50 years, none has affected more people worldwide than this one.1 The current pandemic has forced the global community to consider how everyday actions can affect family, friends, colleagues, and patients. Terms such as testing, contact tracing, infectious transmission, and vaccination have become commonplace. The pandemic has also had a major impact on peoples' understanding of racial and ethnic disparities, professional exposure, business and disease modeling, and the importance of science to society.
This article will focus on how dental professionals can be best served by understanding how to protect themselves, their associates, and their patients. Its aim is to bring dental professionals up to date relative to safe practices and how these practices can be better implemented in a dental office setting. The article will concentrate on COVID-19 testing and address: (1) the biology of the virus; (2) why testing is important; (3) what types of tests are available, the main rationale for each test, and when tests can most effectively be performed; (4) testing assessment; (5) testing in the dental office and reducing anxiety through testing; and (6) regulatory aspects of testing.
SARS-CoV-2 Virus: Brief Overview of the Biology
COVID is an infectious disease initiated by a coronavirus. Unlike bacterial infections, which can colonize surfaces, acquire nutrition and divide, viruses cannot multiply without a viable host. For a virus to flourish it is required to attach to and invade living/dividing cells and then use the cell machinery to replicate.2 A virus will not replicate in cells that are not alive. Severe acute respiratory syndrome (SARS) viral particles are infinitesimally small, and approximately 1 trillion of these tiny particles would fit into one raindrop.
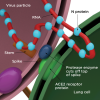
To understand SARS-CoV-2, it is a critically important to appreciate the steps required to initiate disease. SARS-CoV-2 viral infection begins with the interaction of the outward extending spike protein (the corona or crown) with the host cell via interaction of spike with receptors on the surface of the mammalian cell membrane (MCM). The spike protein appears as if it were a lollipop extending from the surface of the virus (Figure 1). Typically, receptors on the surface of the MCM, called "angiotensin-converting enzyme 2 receptors" (ACE2), interact in a lock and key manner with the spike protein of SARS-CoV-2. The spike protein is then attacked by a protease, removing the S1 portion of spike and rendering the S2 portion and the stem of the lollipop active for fusion with the MCM (Figure 2). This fusion opens up a channel into the cell permitting viral RNA affiliated with stabilizing N proteins to enter the cytoplasm of the cell through a process called "endocytosis." This endocytotic/invaginated vesicle (endolysosome) allows viral RNA to move through the cell cytoplasm and enter the rough ribosomal endoplasmic reticulum. Here the viral RNA co-opts ribosomes commissioning them to make and assemble viral proteins by means of RNA-dependent RNA polymerases. N proteins link to viral RNA for stabilization and permit resilient RNA to enter the Golgi complex to further assemble the spike, M, and E proteins formed within Golgi vesicles (Figure 1). These vesicles move toward the inner portion of the outer cell membrane, fuse with the cell membrane, and secrete new complete SARS-CoV-2 virions (complete viral particle) with its spike corona (Figure 3). The process from start to finish is estimated to take 10 hours.3,4
SARS quite possibly affects tongue and nasal epithelium because taste and smell are hallmark symptoms of disease. Thus, it is likely that saliva and the nasopharynx are good sources for viral detection in the early stages of disease. As disease continues, the virus likely migrates to the lung tissue to cause progressive damage, morbidity, and possibly mortality.5
What Is Testing, and What Does It Achieve?
SARS-CoV-2 is a coronavirus that appears to have been passed from bats to pangolins to humans, although much research is still needed to establish that theory.6 The SARS sequence that was originally released from China has permitted testing and vaccine development to progress at an unprecedented pace. Sequencing has allowed research laboratories to develop antigen, antibody, and molecular tests that enable public health workers to identify potential carriers of the virus.7,8
Taking a sample from the nasopharynx, nose, oropharynx, or mouth (saliva) is the most convenient method of testing to identify carriers of the virus.9 Because the disease is thought to be transmitted by aerosol and droplets, masking has been shown as a way of reducing person-to-person transmission of the virus.10 Because aerosol is derived from the nose (sneezing) and mouth (breathing and coughing), testing has focused on samples taken from the nose or mouth. A positive test should result in either isolation or quarantine of the carrier and his or her contacts to limit spread of viral transmission.
Testing Modalities
COVID-19 tests can be divided into two main categories: tests for current infection, and tests for past infection and disease. Testing for current infection can be divided into molecular tests and antigen tests.
Molecular tests are diagnostic tests that detect the genetic signature of the virus. When a sample is collected, the RNA virus is experimentally converted to DNA, which is amplified, typically using RT-PCR (real-time polymerase chain reaction) to make millions of copies of DNA. The DNA binds to a complementary probe that contains a linked fluorescent marker (beacon), the intensity of which is measured by fluorescent light emission detected by spectrophotometry, which indicates the level of SARS-CoV-2 that was collected. This typically requires a laboratory with a PCR machine that amplifies the DNA and a reader that reads the intensity and quantifies the amplified DNA. Among these molecular tests are PCR (polymerase chain reaction), LAMP (loop-mediated isothermal amplification), and NAAT (nucleic acid amplification test). Use of molecular beacons is a highly sensitive way of detecting a signal and the beacons can be multiplexed and designed to indicate both presence of SARS and mutants within the framework of the spike, nucleocapsid, and envelope regions. Molecular tests usually take from an hour to many hours to days to complete and often require expensive laboratory equipment and highly trained technicians. These tests are typically the most accurate (that is, they provide the best sensitivity and specificity, which will be discussed in greater detail later) and detect the virus both at the earliest timepoint and at its lowest level.7,8 Molecular tests typically are the most expensive to run but the most reliable. As with all tests, however, poor sample collection can undermine reliability.9
Antigen tests are diagnostic tests designed to detect one or more of the viral antigens (proteins). The sample is collected from the nasopharynx or anterior nasal region. The sample is then placed in a buffered solution and positioned onto a small well on a plastic hand-held rectangular cartridge. The material in the well flows downstream on a series of stacked pads: a sample pad, conjugation pad, detection pad, and absorbance pad. Each pad has a unique function. The sample pad is for controlled flow. The conjugation pad allows the antigen to associate with a labeled antibody to the designated anolyte (spike protein, for instance) primed to migrate to the detection pad. Here, the immobilized antibody conjugated to spike is seen as a colored line reflecting the presence and level of the anolyte in question. The test is relatively quick (from 10 to 30 minutes) and requires no added equipment or training, although testing can be enhanced by means of cameras, phones, or simple external devices.7 Accuracy and expense are less than molecular testing, although sensitivity and specificity are considerably lower than molecular tests. A test can cost from as little as $5 up to $40 or more. Antigen tests are also dependent on sampling, although sampling is relatively simple and outcomes are best within 5 to 10 days after infection.
A third type of test,antibody tests, are not diagnostic in nature. They detect antibody to one or more of the viral antigens and measure the body's response to the virus. When challenged with the virus, the human body's immune system reacts by creating antibodies, molecules that are specific for an organism's antigens. These antibodies protect the person from reinfection with the virus and, therefore, confer immunity. Vaccines simulate infection, induce antibody responsiveness, and impart protection. Antibodies can be measured by taking peripheral blood samples or with a finger stick that absorbs serum and can then be eluted to be tested for antibody response.11 While antibody infers past infection, it does not automatically imply that the individual is or is not currently infected. An infected person can still form antibodies while still forming viral particles for transmission. Thus, to reiterate, antibody tests are not diagnostic, as it is not possible to know from results if infection is current or past.11 Knowledge of presence of disease or lack thereof is best determined if antibody tests are supplemented by molecular testing. Antibody testing can employ lateral flow (point-of-care [POC]) devices where the antigen (viral protein) is used to capture the serum-derived antibody (IgG, IgM, or IgA), or the testing can be done using an enzyme-linked immunoassay (ELISA), which is more complicated but more quantitative in nature. IgM is typically the earliest antibody detected and usually disappears several days post-infection. IgG usually manifests after IgM but is highly specific for viral proteins and remains present after disease and confers much of the immunity.11,12 POC devices are simpler to use than ELISA, require less equipment, and are less expensive ($10 to $30 per test). They provide best results 15 days or more after infection or about 10 days post-symptoms.
The graph shown in Figure 4 provides context to the course of infection and when particular tests are most effective. Note the significant time differences between infection and disease.13,14 As depicted, although viral RNA and antigen appear around the same time, molecular tests are more reliable because they amplify the viral signal at an earlier timepoint. Antigen tests require a reasonably high viral load to be accurate and usually are accurate concurrent with the onset of symptoms, around 5 days post-infection. Antibody tests are not reliable until day 15+ because it takes that long for the body's immune response to have a high enough level, or titer, of antibodies. Figure 4 represents averages; any individual's COVID-19 disease may vary from the average.
Testing Assessment: Evaluating Test Accuracy
A number of key parameters may be used to evaluate testing:
Sensitivity-This is a measure of how accurate a diagnostic test is for a person who is virus-positive. As stated earlier, molecular tests, while often taking the longest amount of time and costing the most, are the most sensitive tests. Sensitivity is the capability of a testing system to correctly identify those cases that have the disease (true positive rate). Thus, if there are 100 cases that have pneumonia and the test identifies 80, the sensitivity rate is 80%; this means 20 cases in 100 were missed (false negative rate = 20%). When the test fails to confirm a known positive result, this is called a false negative.15,16
Specificity-The inverse of sensitivity, specificity is a measure of how precisely a diagnostic or antibody test correctly indicates a known negative. When a test shows a positive result for a person who isn't actually positive, this is called a false positive. Specificity is the capability of a testing system to identify those cases that do not have the disease (true negative rate or false positive rate). So, if there are 50 cases tested that are negative for disease, and 40 are identified as negative and 10 positive, the specificity rate is 80% and the false positive rate is 20%.
An example paralleling this can be described as follows: Airport security scanners identify all objects that show even the slightest suspicion of being a cause of danger to airport customers or personnel. This means that although these devices show a high level of sensitivity, most often they identify objects that are of no danger to anyone at all. If only 10 out of 100 suspicious items detected by the scanner are actually deemed dangerous, the scanner shows many false positives (90%) and, thus, has a low level of specificity.15,16
Positive predictive value-This value is calculated by determining the number of true positives over the number of true positives plus false positives (ie, the true positives over the total positives).15,16
Accuracy-A formula that utilizes both sensitivity and specificity, accuracy is a single number that indicates the overall efficacy of a test.
Limit of detection-This is the lowest concentration of an analyte that can be reliably (usually with 95% confidence) detected.
Testing in the Dental Office and Its Effect on Relieving Anxiety
Patient and dental healthcare worker safety in a dental office can be increased by patient testing prior to a dental visit and by regular testing of staff workers. Tests can be performed by collecting saliva, nasal, or nasal/pharyngeal specimens and sending the specimens to a commercial laboratory; alternatively, POC testing can be performed in the dental office. Each testing modality has pros and cons, and both dental professionals and patients may have fears and anxiety related to COVID testing.
COVID Anxiety and the Role of Testing
The COVID-19 pandemic has created havoc on the physical health of those affected and the mental health of the general population. Problems such as stress, anxiety, depression, insomnia, denial, anger, and fear have surfaced globally.17 Early in the pandemic (March 2020) the impact of COVID-19 was notable. Perceptions about the likelihood of getting infected, death, and steps to prevent infection were associated with increased mental distress.18 A study conducted between April and September 2020 found high rates of depression (55%) and anxiety (65%) and increased alcohol consumption.19 People of color and sexual and gender minorities were at higher risk of stress.
While living and functioning during a pandemic is stressful for everyone, early studies demonstrated the profound impact on healthcare providers.20,21 Listening sessions with healthcare professionals revealed multiple sources of anxiety, including access to PPE, potential exposure to COVID-19, lack of access to testing, uncertainty of institutional support, childcare accessibility, support for family needs, ability to provide competent care in multiple settings, and lack of access to up-to-date information and communication.22 Psychological and coping responses have been studied in association with past outbreaks, such as H1N1, Ebola, SARS, and Middle East respiratory syndrome (MERS),23 and have included anxiety/fear, depression, anger, guilt, grief, loss, and stigmatization, but also a greater sense of empowerment, compassion toward others, and educating others about the outbreak. Coping strategies included problem-focused coping, seeking social support, avoidance, and positive appraisal of the situation.
In a study of US medical students during the early height of the pandemic, 30.6% screened positive for anxiety and 24.3% for depression, with significantly higher scores for female respondents.24 These numbers were higher when compared with pre-pandemic studies of medical students. In one study, health literacy was found to protect medical students from fear, suggesting that knowledge and information can be a valuable intervention to reduce anxiety.25 Levels of anxiety via self-report were higher in dental hygienists (25.7%)26 than dentists (14.8%),20 suggesting that being in control may have equated to lower anxiety; however, these data were collected in two different studies and may not be comparable.
In a study that focused on the life experiences of essential workers during the early stages of the COVID-19 pandemic there was a noted negative impact on daily life, interactions with others, stress levels, and enjoyment of life, yet also a positive impact on caring about one another, self-care, and exercise.27 Among essential workers, dental workers were significantly less afraid than non-dental healthcare and non-healthcare providers. Notably, knowing the results of an antibody test was reported to decrease the level of stress and anxiety. Having the antibody test delivered in a dental setting was met with strong satisfaction and acceptability. This suggests that widespread testing and specifically testing in dental care settings may help to alleviate stress and anxiety associated with a pandemic.
There is a lack of both quantitative and qualitative evidence from studies carried out during or after disease epidemics and pandemics that can inform the selection of interventions that are beneficial to the resilience and mental health of frontline workers.28 In a systematic review of interventions utilized during an outbreak/pandemic there was no specific inclusion of the use of testing to address anxiety. Interventions studied included training, structure and communication, and counseling and psychological services. It is possible that testing could fall within an area of training and/or communication, but clearly more definitive studies are necessary to delineate the specific role that testing can play.
In a study of obstetric patients and labor and delivery personnel during the COVID-19 pandemic, the perceptions of a university testing program were determined.29 In the obstetric patients who were hospitalized 72.4% said testing did not change their anxiety. They acknowledged that this was likely because they were hospitalized and remained at risk for infection. In contrast, the labor and delivery personnel reflected on the impact of the universal testing program and 54.5% expressed appreciation for universal testing. These authors concluded that a testing program improved employee satisfaction and reduced anxiety.
The following sections describe laboratory-based testing and in-office POC testing and how they can be conducted in dental practice.
Laboratory-Based Testing
Laboratory-based testing requires that a patient or dental healthcare worker go to a testing site for collection of their saliva and/or nasal or nasal/pharyngeal specimen. Alternatively, the patient can come to the dental office for the specimen to be collected, or the specimen can be collected at home and then sent overnight to the laboratory. Laboratory-based PCR testing is generally more accurate than antigen POC tests, but results are delayed as it takes time for the specimen to arrive at the lab and be processed. Thus, PCR sample results received in the dental office can be several days old. However, with PCR testing, offices do not have to allocate space or personnel to the testing process, nor do they have to register as a Clinical Laboratory Improvement Amendments (CLIA)-Waived facility (see "Regulatory Terms Related to COVID-19 Testing" sidebar, p.294). Further, there is generally no requirement that the office report results to the state health department because the commercial laboratory will handle reporting requirements.17
Point-of-Care Testing
POC testing can be done as a patient enters the dental office or is seated in the operatory. When a POC antigen test is performed, appropriate space in needed to obtain the nasal specimen from the patient. Patients who arrive at the dental office by automobile could be asked to wait in their cars until the test has been completed. Otherwise, sufficient space is required to enable a patient to wait safely. POC tests done in a dental office generally take 15 minutes to complete and must be CLIA-Waived. These tests generally are antigen tests; some require the use of an instrument to read test results from the test strip or test cartridge, while others are self-contained systems that do not necessitate a reader.17
Most states require that CLIA-Waived COVID test results be reported to the state health department by the facility performing the test. To do this reporting, the dental office must designate one or more individuals to be registered with the state, be trained to use the state reporting system, and have an active username and passcode for accessing the state's reporting system.
To summarize this section on COVID-19 anxiety and the role of testing in the dental office,a body of literature exists on the role of tests as more than just tools for diagnosis.30-32 Tests offer healthcare providers reassurance in the face of uncertainty and can be a benefit for patients. Patients whose requests for tests are fulfilled have higher satisfaction with their healthcare providers, while denial of tests leads to lower patient satisfaction.33 Chairside medical screening by dentists is seen favorably by patients and providers and can be used to effectively identify patients who would benefit from medical follow-up.34,35
Conclusions: Beyond COVID-19
A myriad of tests that are CLIA-Waived are available and appropriate at the POC level. Dentists with a CLIA-Waived certificate and whose state practice act permits point-of-care testing can perform an increasing panel of tests. These include tests for both medical/systemic (eg, flu A/B) and dental conditions (eg, periodontal disease, oral cancer, etc). Dentistry is unique in its ability to provide a valuable, sometimes life-saving function. Many people do not visit their physicians until they are feeling poorly, having waited until they may be very ill and possibly beyond treatment that can modify their condition. These same individuals, however, may see their hygienist and/or dentist at least twice yearly. Dental professionals can play an ever-increasing critical role in their patient's health by using easy-to-administer, point-of-care, safe and rapid CLIA-Waived tests that are coming online for a wide array of conditions. Practitioners who have applied for and received a CLIA-Waived certificate during this public health emergency are fully qualified to participate in a service that can be provided only by dental professionals.36
This crisis, which is otherwise overwhelmingly devastating, can offer an opportunity for dental professionals to improve the public health outcome for many patients, including those who might ordinarily go unnoticed and untreated.37 Furthermore, evidence also suggests that testing for all parts of society in the wake of this pandemic and for the immediate future will be of value in relieving anxiety in the general population.
About the Authors
Bruce Lieberthal, DMD
Vice President and Chief Innovation Officer, Henry Schein, Inc., Melville, New York
Laurie K. McCauley, DDS, MS, PhD
Dean, William K. and Mary Anne Najjar Professor of Periodontics, University of Michigan School of Dentistry, Ann Arbor, Michigan
Cecile A. Feldman, DMD, MBA
Dean and Professor of Dentistry, Rutgers School of Dental Medicine, Rutgers University, Newark, New Jersey
Daniel H. Fine, DMD
Professor and Chair, Department of Oral Biology, Rutgers School of Dental
Medicine, Senior Associate Dean, School of Graduate Studies, Rutgers University, Newark, New Jersey
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Jiang Y, Jiang X, Tong W, Zhou J. Quantitative analysis and mathematic modeling of the global outbreak of COVID-19. J Infect Dev Ctries. 2020;31;14(10):1106-1110.
2. Agut H, Fillet AM, Calvez V. Qu'est-ce qu'un virus? [What is a virus?] [in French]. Rev Prat. 1997;47(6):602-607.
3. Davidson AD, Williamson MK, Lewis S, et al. Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein. Genome Med. 2020;12(1):68.
4. Hemmat N, Asadzadeh Z, Ahangar NK, et al. The roles of signaling pathways in SARS-CoV-2 infection; lessons learned from SARS-CoV and MERS-CoV. Arch Virol. 2021;166(3):675-696.
5. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793.
6. Wahba L, Jain N, Fire AZ, et al. An extensive meta-metagenomic search identifies SARS-CoV-2-homologous sequences in pangolin lung viromes. mSphere. 2020;5(3):e00160-20.
7. Miscio L, Olivieri A, Labonia F, et al. Evaluation of the diagnostic accuracy of a new point-of-care rapid test for SARS-CoV-2 virus detection. J Transl Med. 2020;18(1):488.
8. Chong Y, Tani N, Ikematsu H, et al. Genetic testing and serological screening for SARS-CoV-2 infection in a COVID-19 outbreak in a nursing facility in Japan. BMC Infect Dis. 2021;21(1):263.
9. Rao M, Rashid FA, Sabri FS, et al. Comparing nasopharyngeal swab and early morning saliva for the identification of SARS-CoV-2. Clin Infect Dis. 2020;ciaa1156. doi: 10.1093/cid/ciaa1156.
10. Chu DK, Akl EA, Duda S, et al, COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973-1987.
11. Theel ES, Slev P, Wheeler S, et al. The role of antibody testing for SARS-CoV-2: Is there one? J Clin Microbiol. 2020;58(8):e00797-20.
12. Randad PR, Pisanic N, Kruczynski K, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. medRxiv [preprint]. 2020;2020.05.24.20112300. Update in: J Clin Microbiol. 2020;59(1):e02204-20.
13. Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv [preprint]. 2020;2020.04.14.20065771. Update in: Nat Commun. 2020;11(1):4704.
14. Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17(7):773-775.
15. Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56(6):e00252-18.
16. Mak GC, Lau SS, Wong KK, et al. Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus. J Clin Virol. 2020;133:104684.
17. de Almeida Barros Mourao CF, Javid K, Bastos Barbosa I. How can we reduce the risks of SARS-CoV-2 (COVID-19) for dentists and their patients? Evid Based Dent. 2020;21(2):50-51.
18. Torales J, O'Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020;66(4):317-320.
19. Holingue C, Kalb LG, Riehm KE, et al. Mental distress in the United States at the beginning of the COVID-19 pandemic. Am J Public Health. 2020;110(11):1628-1634.
20. Estrich CG, Gurenlian JR, Battrell A, et al. COVID-19 prevalence and related practices among dental hygienists in the United States. J Dent Hyg. 2021;95(1):6-16.
21. Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976.
22. Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID-19 pandemic: a cross-sectional study. Psychiatry Res. 2020;288:112936.
23. Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133-2134.
24. Chew QH, Wei KC, Vasoo, S, et al. Narrative synthesis of psychological and coping responses towards emerging infectious disease outbreaks in the general population: practical considerations for the COVID-19 pandemic. Singapore Med J. 2020;61(7):350-356.
25. Halperin SJ, Henderson MN, Prenner S, Grauer JN. Prevalence of anxiety and depression among medical students during the Covid-19 pandemic: a cross-sectional study. J Med Educ Curric Dev. 2021;8:2382120521991150.
26. Nguyen HT, Do BN, Pham KM, et al. Fear of COVID-19 scale-associations of its scores with health literacy and health-related behaviors among medical students. Int J Environ Res Public Health. 2020;17(11):4164.
27. Fontana M, McCauley L, Fitzgerald M, et al. Impact of COVID-19 on life experiences of essential workers attending a dental testing facility. JDR Clin Trans Res. 2021;6(1):24-39.
28. Estrich CG, Mikkelsen M, Morrissey R, et al. Estimating COVID-19 prevalence and infection control practices among US dentists. J Am Dent Assoc. 2020;151(11):815-824.
29. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11:CD013779.
30. Bender WR, Srinivas S, Coutifaris P, et al. The psychological experience of obstetric patients and health care workers after implementation of universal SARS-CoV-2 testing. Am J Perinatol. 2020;37(12):1271-1279.
31. van der Weijden T, van Bokhoven MA, Dinant GJ, et al. Understanding laboratory testing in diagnostic uncertainty: a qualitative study in general practice. Br J Gen Pract. 2002;52(485):974-980.
32. Watson J, de Salis I, Banks J, Salisbury C. What do tests do for doctors? A qualitative study of blood testing in UK primary care. Fam Pract. 2017;34(6):735-739.
33. Fenton JJ, Magnan EM, Jerant A, et al. Patient characteristics associated with making requests during primary care visits. J Am Board Fam Med. 2019;32(2):201-208.
34. Jerant A, Fenton JJ, Kravitz RL, et al. Association of clinician denial of patient requests with patient satisfaction. JAMA Intern Med. 2018;178(1):85-91.
35. Greenberg BL, Kantor ML, Jiang SS, Glick M. Patients' attitudes toward screening for medical conditions in a dental setting. J Public Health Dent. 2012;72(1):28-35.
36. Clinical Laboratory Improvement Amendments (CLIA) Laboratory Guidance During COVID-19 Public Health Emergency. Baltimore, Md: US Dept of Health and Human Services, Centers for Medicare and Medicaid Services; March 26, 2020. Ref: QSO-20-21-CLIA.
37. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)-HCFA. Final rule with comment period. Fed Regist. 1992;57(40):7002-7186.
38. Mitchell SL, St George K, Rhoads DD, et al. Understanding, verifying, and implementing emergency use authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol. 2020;58(8):e00796-20.
39. Greenberg BL, Glick M. Providing health screenings in a dental setting to enhance overall health outcomes. Dent Clin North Am. 2018;62(2):269-278.