You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
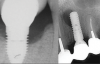
Over the past 30 years, multiple robust experimental and clinical studies have proven Bränemark's osseointegration principle of titanium dental implants. However, their widespread use has resulted in numerous types of complications. Even when the most stringent rules for sterility are followed, optimal surgical planning is carried out, and careful patient selection/preparation is practiced, some implants will fail in either their integration or prosthetic phase. An implant periapical lesion is a rare complication that may occur following implant placement, and case reports have suggested that such lesions are a possible reason for early failure of an endosseous implant.1-3 Ailing and failing implants can be treated to try to preserve them; however, failed implants must be removed because they are nonfunctional and bone loss will continue. The apical lesion described in this article is considered to be a different category, as typical pocket pathology is unrelated to this condition (Figure 1).
The implant periapical lesion, also called "apical peri-implantitis" or "retrograde peri-implantitis," is defined as a clinically symptomatic periapical lesion that can develop after the placement of an implant. The coronal portion of the implant maintains a normal bone-to-implant interface.4,5 McAllister et al described the first cases of this condition in 1992, followed by Sussman and Moss in 1993.6,7
This review is intended to provide an overview of the literature on the prevalence, etiology, and diagnosis of these lesions to assist clinicians in understanding the pathology and offer different strategies for prevention and treatment. A diagnostic clinical and radiographical approach is proposed for the identification of this entity in its different stages and to establish the appropriate treatment approach accordingly.
Literature Search
A literature search was performed in PubMed and Google Scholar for articles on RPI published in English. The search was performed in 2020 using three different search terms, which yielded 57 articles related to RPI: "apical lesion implant," "retrograde peri-implantitis," and "periapical lesion implant." All article abstracts were reviewed, and 37 articles were selected for inclusion in this literature review. This analysis included any case report or clinical trial that attempted to treat or rescue an implant diagnosed with RPI, and any treatment modality was allowed, including nonsurgical treatment, surgical debridement, and/or apicoectomy performed on the affected implant.
Results
Prevalence
The prevalence of retrograde peri-implantitis is low. Reiser and Nevins found 10 cases in 3,800 implants placed (0.26%).8 In a retrospective study involving 539 implants, Quirynen et al obtained a prevalence of 1.6% in the maxilla and 2.7% in the mandible, with all cases diagnosed before the second-stage surgery.9 Incidence of RPI is reported to increase to 7.8% when teeth adjacent to the implant site have a previous history of root canal therapy.10
Etiology
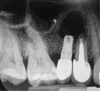
Among the factors associated with the appearance of this pathology are: contamination of the implant surface or surgical bed,11,12 overheating of bone during implant bed preparation,11,13 preparation of an implant bed that is longer than the implant itself,8 pre-existing bone disease,1 presence of residual root fragments or foreign bodies,8,11 premature loading causing bony microfractures,14 and implant placement in proximity to an infected maxillary sinus.14 Some studies have suggested the most likely cause is endodontic pathology of the tooth replaced by the implant or adjacent tooth.9,10 Figure 2 shows an intraosseous tract within the mandible from a periapical lesion of a tooth leading to RPI of the adjacent implant.
Ayangco and Sheridan published three cases of implant periapical lesions in patients in whom failure of apical surgery of the teeth had occurred before implant placement.1 According to the authors, despite curettage of the sockets and a prolonged waiting period before implant insertion, bacteria could remain in the bone causing subsequent development of lesions in the implants. Brisman et al attributed the failure of four implants to the existence of adjacent endodontically treated teeth, which were asymptomatic and showed no radiographic signs of pathology.15 Sussman classified the lesions into two types: implant to tooth (type I), when the neighboring tooth is injured during implant drilling; and tooth to implant (type II), when the lesion occurs due to contamination of the implant from an apical lesion of the adjacent tooth.16 Finally, Balshi et al suggested that the etiology of this process is multifactorial, and they were unable to confirm or reject any of the aforementioned hypotheses.17
Diagnosis
Based on case reports analyzed, RPI is diagnosed between 1 week and 4 years after implant placement.10,18 Other studies reported that RPI lesions were diagnosed within 6 months after implant placement.9,17,19
The diagnosis of periapical implant lesions can be made clinically and radiographically. Typical symptoms and clinical signs that may appear are pain, erythema, swelling, suppuration, and fistulas; in a radiograph a well- or ill-defined implant periapical radiolucency can be identified in some cases. Reiser and Nevins classified implant periapical lesions as inactive (ie, not infected) or active (ie, infected).8 Determining the stage of the lesion is helpful in applying the best treatment option. Several scenarios are possible:
Scenario 1: In this scenario the RPI is the clinical inactive and asymptomatic form. It is diagnosed because of the presence of a radiolucency around the apex of the implant. This radiolucency might be only an apical scar caused by vertical overpreparation of the implant bed or by bone necrosis due to overheating during implant insertion. Inactive lesions do not necessarily require treatment unless the radiolucency grows in size; these lesions should only be controlled and monitored radiographically.
Scenario 2: In this scenario the lesion is clinically symptomatic and acute. This condition requires treatment in order to avoid the progression of peri-implant bone destruction. Besides periapical radiolucency, which may or may not be present, other clinical signs and symptoms may appear, including pain on palpation, gingival reddening, soft-tissue swelling of the gingival mucosa, and, in some cases, presence of pus or a fistulous tract in the region of the implant apex.
In some Scenario 2 cases, there may be acute apical peri-implantitis without the presence of pus. In such a case, there is an acute inflammatory infiltrate that is clinically characterized by the presence of acute spontaneous and localized pain, which does not increase with percussion. The mucosa can be swollen and painful and implant percussion produces a metallic sound. In most such cases no changes in bone density can be seen in the radiograph around the implant apex.
Progression can lead to a suppurated acute apical peri-implantitis or apical abscess, where a pus collection is formed around the implant apex. Clinical symptoms are similar to that of the non-suppurated stage, but an implant periapical radiolucency is usually observed. The pus collection looks for least-resistant drainage pathways and destroys bone around the implant apex; once the drainage pathways are created a subacute or chronic apical peri-implantitis is established. If the coronal bone-implant junction is well consolidated, a fistulous tract develops from the implant apex through the buccal cortical plate. If the coronal bone-implant junction is not well consolidated, this will be the least-resistant drainage pathway; the peri-implant bone will be destroyed coronally and the implant will be lost as a result. In this chronical stage the symptoms are not marked; there may be a fistulous tract, a buccal abscess, or suppuration around the implant neck. Depending on the progress of the process the implant may be mobile, and a peri-implant bone destruction along the body of the implant may be seen in the radiograph and should be classified in regard to the implant length. The author suggests classifying the amount of peri-implant bone loss in correlation to the implant length according to Shah et al.20
The clinician must be careful when making this assessment because 2-dimensional (2D) periapical radiographs do not always show the actual size of an intrabony defect. These kinds of defects can be identified only when the junctional area is involved. Thus, it is possible that some periapical pathologies may not be recognized on 2D radiographs, which is a limiting factor in the diagnosis. Cone-beam computed tomography (CBCT) can be used to overcome this limitation.
Treatment
The correct diagnosis of apical peri-implant lesions in their early stages allows for early treatment and can prevent implant failure.10,21 However, treatment of periapical peri-implantitis is still only empirical. The author's search of the literature led to predominantly case reports of possible treatment options. To the best of the author's knowledge, to date no consensus has been published for treatment of RPI. Clinicians often are confounded by its clinical and radiographic presentation and treat it on the basis of empirical data. Nonsurgical and surgical treatment strategies with preservation of the implant (Table 1) as well as surgical treatment with removal of the implant (Table 2) have been reported in the literature and can be applied.
Nonsurgical Approach With Preservation of the Implant
A periapical implant radiolucency can be a casual finding during a routine radiographic assessment. If the implant is asymptomatic and the diameter of the radiolucent area is small, it is not necessary to treat the lesion. Overpreparation of the implant bed is the most probable cause of the radiolucency, and only regular radiographic controls should be performed. If, as seen in any of the controls, the radiolucency increases in size or the patient develops symptoms, a surgical treatment is then recommended. In this regard, Waasdorp and Reynolds suggested that asymptomatic implant periapical lesions could resolve by antibiotic therapy only without any surgical intervention, as a lesion of this type was fully resolved after treatment with antibiotics (amoxicillin 500 mg three times a day for 10 days).22 According to those authors the etiology of the apical peri-implantitis was uncertain. Other reports have suggested that in the presence of inactive or asymptomatic lesions no treatment is indicated.8
Some published case series have reported that initial treatment with antibiotics was not effective to control symptomatic or active lesions, which required surgical access.23,24 Romanos et al25 concluded in their review that antibiotic treatment alone is not effective (Table 3).2,11,17,18,22,24,26-32
Surgical Approach With Preservation of the Implant
Apical implant surgery-open-flap, implant surface decontamination, apical degranulation, with or without defect management. If a patient presents with acute pain that is well localized on the apex of the implant and gingival swelling, the presence of an acute apical peri-implantitis must be suspected and an apical implant surgical treatment should be considered. If an apical radiolucent area is identified, it is usually accompanied by bone destruction around the implant body, and soft-tissue signs such as a fistulous tract or vestibular abscess are present. In these cases, if the periapical radiolucency is less than 50% of the implant length, the clinician must ensure that the stability of the implant has not been compromised.20 The most studied treatment of implant periapical lesions with no associated implant mobility is implant apical surgery.33
A common approach is to curettage the lesion and irrigate with saline solution.1,9,23 Several agents, including chlorhexidine19,23,29 and tetracycline pastes,1,17,19 have been applied for decontamination of the implant surface, but there is no evidence of the efficiency of any of them. Bone regeneration materials have been used and have sometimes been accompanied by tissue regeneration barriers or platelet-rich fibrin (PRF) depending on the defect size to achieve complete bone regeneration of the defect.2,9,18
Open-flap with apical implant resection and degranulation, with or without defect management, with or without root canal therapy/apicoectomy of adjacent teeth. Other reports have suggested resecting the implant apex in cases in which total removal of granulation tissue is not assured, and when the maxillary sinus or nasal cavity is involved.1,17,24 Tözüm et al even performed root canal retreatment or periapical surgery if the adjacent tooth was endodontically treated (Table 3).18 If the lesion is secondary to a primary endodontic lesion from an adjacent tooth, endodontic management of the offending tooth should be performed. This should be followed up for several months. Closed periapical implant lesions have high healing potential and demonstrate complete resolution of periapical lesion. If resolution occurs, the patient should continue to be monitored. If the lesion shows no sign of resolution or shows signs of progressing, it should at this point be managed as a primary apical implant lesion.20
Resection of the implant allows for the complete removal of the lesion and the contaminated implant surface while leaving enough integrated implant length to support the restoration and the stability of the implant. It is crucial to treat the implant before the lesion spreads coronally. In general, the healing potential of such lesions after intervention may be higher than in conventional peri-implantitis, where the crestal bone is involved. This has been attributed to the fact that after completion of therapy, a closed environment is obtained and healing occurs relatively undisturbed.34
The author's own experience is in accordance with the literature and has demonstrated that the apical implant resection procedure is an effective treatment method to maintain an implant with an apical lesion, as it provides a stable state of osseointegration without further complication. Once diagnosed, the lesion should be treated aggressively rather than by observation and conservative management.35 The following case report demonstrates this approach.
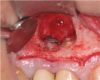
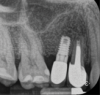
Report of a case. A 45-year-old male patient was referred with pain, redness, and swelling around an implant in the position of the maxillary right second premolar. The implant had been in clinical function for 3 years. The patient had a history of endodontic treatment and tooth loss and subsequent implant treatment in the region. Radiographic examination showed a radiolucency around the apical portion of the implant and the adjacent tooth. The implant was clinically stable and the neighboring tooth No. 4 was considered to have a periapical lesion. Initially, the patient received antibiotics (clindamycin 300 mg, three times daily) and was followed up for 2 weeks. After this, the patient was scheduled for surgical exploration of the implant area. Treatment comprised elevation of a full-thickness flap, curettage of the apical lesion, irrigation with saline, and removal of the apical portion of the implant and apicoectomy of the adjacent tooth (Figure 3 and Figure 4), including a guided bone regeneration (GBR) procedure with a cancellous particulate allograft and 20 mm x 30 mm pericardium membrane. Granulation tissue was sent for histopathology analysis, which revealed a periapical inflammatory infection. At the 4-month follow-up increased radiographic bone density could be observed consistent with the formation of bone not only around the implant apex but also along the root of tooth No. 5 (Figure 5).
Surgical Approach With Removal of the Implant
If an apical peri-implant lesion demonstrates advanced bone loss (ie, more than 50%) implant mobility must be carefully assessed. If mobility is absent, treatment could consist of an apical implant resection as described above but implant removal should be strongly considered.
If implant mobility is evident, an atraumatic approach (preferably countertorque or counterscrew technique)36,37 is the only treatment option. After implant removal, if sufficient bone remains to achieve good implant stability and the decontamination of the site is thorough, immediate wide-diameter implant placement can be performed. However, if sufficient bone is not present, treatment must comprise GBR followed by delayed implant placement.
Prognosis
The current literature lacks sufficient data to predict evidence-based implant survival rates of different treatment modalities of RPI. Most reports are of only few clinical cases, and it remains difficult to determine the prognosis of implants treated with periapical surgery. Romanos et al studied the prognosis of implant apical lesions after reviewing all cases published up to December 2007; 75% of the implants diagnosed with periapical lesions survived after treatment, with follow-up periods ranging between 4 months and 7 years.25 In another study, the apical resection of the implant with or without GBR/PRF demonstrated the highest success rate (97.4%), with no further complications.17
Conclusion
The prevalence of RPI is low. To date, there is no consensus about the exact etiology. Although the lesion is mostly associated with endodontic pathology of adjacent teeth, several factors might act together, pointing to a multicausality. Proper prevention of RPI could be accomplished by careful preoperative assessment of the implant bed and adjacent teeth. Diagnosis of an implant periapical lesion should be based on both clinical and radiological findings in order to apply the best treatment strategy. Treatment recommendations range from conservative approaches, such as follow-up and antibiotic therapy only, to implant surface detoxification and surgical therapies. Apical resection of the implant with or without GBR/PRF removes only the involved portion of the implant, thereby maintaining the osseointegrated portion of the implant and the prosthesis, and has demonstrated the highest success rate with no further complication. Mobile implants with clinical symptoms and/or more than 50% bone loss require an atraumatic removal of the implant that may include GBR procedures and either immediate or delayed implant placement.
Acknowledgment
The author thanks Nadia Basharat, BDS, for assisting in the literature search and formulating the information in Table 1 and Table 2.
About the Author
Thomas G. Wiedemann, MD, PhD, DDS
Clinical Assistant Professor, Department of Oral and Maxillofacial Surgery, New York University College of Dentistry, New York, New York
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Ayangco L, Sheridan PJ. Development and treatment of retrograde peri-implantitis involving a site with a history of failed endodontic and apicoectomy procedures: a series of reports. Int J Oral Maxillofac Implants. 2001;16(3):412-417.
2. Bretz WA, Matuck AN, de Oliveira G, et al. Treatment of retrograde peri-implantitis: clinical report. Implant Dent. 1997;6(4):287-290.
3. Flanagan D. Apical (retrograde) peri-implantitis: a case report of an active lesion. J Oral Implantol. 2002;28(2):92-96.
4. Quirynen M, Gijbels F, Jacobs R. An infected jawbone site compromising successful osseointegration. Periodontol 2000. 2003;33:129-144.
5. Lindeboom JA, Tjiook Y, Kroon FH. Immediate placement of implants in periapical infected sites: a prospective randomized study in 50 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(6):705-710.
6. McAllister BS, Masters D, Meffert RM. Treatment of implants demonstrating periapical radiolucencies. Pract Periodontics Aesthet Dent. 1992;4(9):37-41.
7. Sussman HI, Moss SS. Localized osteomyelitis secondary to endodontic-implant pathosis. A case report. J Periodontol. 1993;64(4):306-310.
8. Reiser GM, Nevins M. The implant periapical lesion: etiology, prevention, and treatment. Compend Contin Educ Dent. 1995;16(8):768-772.
9. Quirynen M, Vogels R, Alsaadi G, et al. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin Oral Implants Res. 2005;16(5):599-608.
10. Zhou W, Han C, Li D, et al. Endodontic treatment of teeth induces retrograde peri-implantitis. Clin Oral Implants Res. 2009;20(12):1326-1332.
11. Piattelli A, Scarano A, Balleri P, Favero GA. Clinical and histologic evaluation of an active "implant periapical lesion": a case report. Int J Oral Maxillofac Implants. 1998;13(5):713-716.
12. Chaffee NR, Lowden K, Tiffee JC, Cooper LF. Periapical abscess formation and resolution adjacent to dental implants: a clinical report. J Prosthet Dent. 2001;85(2):109-112.
13. Esposito M, Hirsch J, Lekholm U, Thomsen P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. Int J Oral Maxillofac Implants. 1999;14(4):473-490.
14. Scarano A, Di Domizio P, Petrone G, et al. Implant periapical lesion: a clinical and histologic case report. J Oral Implantol. 2000;26(2):109-113.
15. Brisman DL, Brisman AS, Moses MS. Implant failures associated with asymptomatic endodontically treated teeth. J Am Dent Assoc. 2001;132(2):191-195.
16. Sussman HI. Periapical implant pathology. J Oral Implantol. 1998;24
(3):133-138.
17. Balshi SF, Wolfinger GJ, Balshi TJ. A retrospective evaluation of a treatment protocol for dental implant periapical lesions: long-term results of 39 implant apicoectomies. Int J Oral Maxillofac Implants. 2007;22(2):267-272.
18. Tözüm TF, Sençimen M, Ortakoğlu K, et al. Diagnosis and treatment of a large periapical implant lesion associated with adjacent natural tooth: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(6):132-138.
19. Chan HL, Wang HL, Bashutski J, et al. Retrograde peri-implantitis: a case report introducing an approach to its management. J Periodontol. 2011;82(7):1080-1088.
20. Shah R, Thomas R, Kumar ABT, Mehta DS. A radiographic classification for retrograde peri-implantitis. J Contemp Dent Pract. 2016;17(4):313-321.
21. Peñarrocha-Diago M, Boronat López A, Lamas Pelayo J. Update in dental implant periapical surgery. Med Oral Patol Oral Cir Bucal. 2006;11(5):
E429-E432.
22. Waasdorp J, Reynolds M. Nonsurgical treatment of retrograde peri-implantitis: a case report. Int J Oral Maxillofac Implants. 2010;25(4):831-833.
23. Peñarrocha-Diago M, Boronat-Lopez A, García-Mira B. Inflammatory implant periapical lesion: etiology, diagnosis, and treatment - presentation of 7 cases. J Oral Maxillofac Surg. 2009;67(1):168-173.
24. Dahlin C, Nikfarid H, Alsén B, Kashani H. Apical peri-implantitis: possible predisposing factors, case reports, and surgical treatment suggestions. Clin Implant Dent Relat Res. 2009;11(3):222-227.
25. Romanos GE, Froum S, Costa-Martins S, et al. Implant periapical lesions: etiology and treatment options. J Oral Implantol. 2011;37(1):53-63.
26. Park SH, Sorensen WP, Wang HL. Management and prevention of retrograde peri-implant infection from retained root tips: two case reports. Int J Periodontics Restorative Dent. 2004;24(5):422-433.
27. Peñarrocha-Diago M, Maestre-Ferrín L, Peñarrocha-Oltra D, et al. Inflammatory implant periapical lesion prior to osseointegration: a case series study. Int J Oral Maxillofac Implants. 2013;28(1):158-162.
28. Zhou Y, Cheng Z, Wu M, et al. Trepanation and curettage treatment for acute implant periapical lesions. Int J Oral Maxillofac Surg. 2012;41(2):171-175.
29. Ataullah K, Chee LF, Peng LL, Lung HH. Management of retrograde peri-implantitis: a clinical case report. J Oral Implantol. 2006;32(6):308-312.
30. Nedir R, Bischof M, Pujol O, et al. Starch-induced implant periapical lesion: a case report. Int J Oral Maxillofac Implants. 2007;22(6):1001-1006.
31. Kutlu HB, Genc T, Tozum TF. Treatment of refractory apical peri-implantitis: a case report. J Oral Implantol. 2016;42(1):104-109.
32. Mohamed JB, Alam MN, Singh G, Chandrasekaran SC. The management of retrograde peri-implantitis: a case report. J Clin Diagn Res. 2012;6(9):1600-1602.
33. Temmerman A, Lefever A, Teughels W, et al. Etiology and treatment of periapical lesions around dental implants. Periodontol 2000. 2014;66(1):247-254.
34. Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15(6):348-381.
35. Mohamed JB, Shivakumar B, Sudarsan S, et al. Retrograde peri-implantitis. J Indian Soc Periodontol. 2010;14(1):57-65.
36. Solderer A, Al-Jazrawi A, Sahrmann P, et al. Removal of failed dental implants revisited: questions and answers. Clin Exp Dent Res. 2019;5(6):712-724.
37. Roy M, Loutan L, Garavaglia G, Hashim D. Removal of osseointegrated dental implants: a systematic review of explantation techniques. Clin Oral Investig. 2020;24(1):47-60.
ADDITIONAL SUGGESTED READING
Chang LC, Hsu CS, Lee YL. Successful medical treatment of an implant periapical lesion: a case report. Chang Gung Med J. 2011;34(1):109-114.
Lefever D, Van Assche N, Temmerman A, et al. Aetiology, microbiology and therapy of periapical lesions around oral implants: a retrospective analysis. J Clin Periodontol. 2013;40(3):296-302.
Oh TJ, Yoon J, Wang HL. Management of the implant periapical lesion: a case report. Implant Dent. 2003;12(1):41-46.
Palma-Carrió C, Maestre-Ferrín L, Peñarrocha-Oltra D, et al. Risk factors associated with early failure of dental implants. A literature review. Med Oral Patol Oral Cir Bucal. 2011;16(4):e514-e517.
Rosendahl K, Dahlberg G, Kisch J, Nilner K. Implant periapical lesion. A case series report. Swed Dent J. 2009;33(2):49-58.
Sussman HI. Implant pathology associated with loss of periapical seal of adjacent tooth: clinical report. Implant Dent. 1997;6(1):33-37.