You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Contemporary dental implant therapy is grounded in foundational studies in which endosseous dental implants were placed into residual alveolar bone of the parasymphyseal mandible and premaxilla. The reported success of implants guided a new means of rehabilitating the edentulous patient.1 Bränemark's team subsequently demonstrated that success was possible using this approach even when encountering relatively marked alveolar bone resorption, which dictated the use of shorter dental implants.2 The primary guiding principles for dental implant success included an assessment of bone quality and bone quantity, which was classified simply according to Lekholm and Zarb.3 Implicit in the classification was the notion that osseointegration realization was dependent on the extent of bone-to-implant contact and that this achievement was favored in conditions of higher bone quality and volume. A second early guiding principle was that dental implant success was biomechanically dependent on the avoidance of interfacial bone overload through the assurance of axial loading of implants. Mutually parallel implant placement oriented perpendicular to the occlusal (and prosthetic) plane was considered crucial to successful implant therapy. These principles guided early successes.
When confronted with patients who lacked sufficient bone quality and quantity to support implants of sufficient length, clinicians developed bone grafting techniques to alter the bone quantity, with a presumption of improved quality, and enable placement of larger/longer dental implants. Interpositional bone grafting and onlay bone grafting, using autogenous bone, represented the first successful demonstrations of altering bone volume for implant therapy.4 These approaches first utilized iliac crest donor sites but subsequently involved the use of oral donor sites, including the mandibular ramus or chin, for onlay grafting. Studies throughout the 1980s and 1990s demonstrated the success of autogenous bone grafting procedures to support implants for the rehabilitation of individuals with resorbed alveolar bone.
Additionally, sinus grafting was introduced as a means of increasing bone volume for the pneumatized or resorbed posterior maxilla. Widely investigated throughout the 1990s, sinus grafting remains a highly successful procedure with limited morbidity that is associated with high dental implant survival.5 The treatment of the resorbed posterior mandible (which is beyond the scope of this article) has been more challenging. Vertical ridge augmentation of the posterior mandible is an often-reported treatment, but it has been found to have greater morbidity and more variable outcomes than other grafting approaches.6 While autogenous bone is considered the benchmark for oral bone grafting procedures, alternative solutions have been introduced, including synthetic, xenogenic, and allogenic materials. These bone grafting materials have demonstrable success when applied to sinus graft and horizontal augmentation procedures and are a prominent part of current dental implant therapies. Adjuvant tools to aid in the volumetric enhancement of alveolar bone grafting have included various resorbable and nonresorbable barrier membranes, tenting pins/screws, and titanium mesh. Experimental approaches involve 3-dimensional design and printing of mesh and bone graft materials.7
Given this continued development and the multiple techniques and materials that may be applied to the augmentation of the resorbed alveolus, the extensive spectrum of approaches would seem to indicate that there remains no defined consensus or "best" approach to the augmentation of resorbed alveolar bone for the purpose of enhancing dental implant therapy.
The success of bone grafting procedures for alveolar bone augmentation is difficult to gauge8 principally because a surrogate measure of success, implant survival, often is used to define bone grafting success. Few studies have meaningfully reported on the volumetric bone outcomes following alveolar bone regeneration for dental implant therapy, and more recent studies have focused on horizontal volumetric changes for single-tooth implants. A key question that has emerged regarding bone grafting procedures and dental implant therapy is, "What is the relative success of implants placed in grafted bone sites compared to implants placed in nongrafted sites?"
Risks of Implant Placement in Grafted Bone
Growing evidence suggests that implant survival or success rates are marginally lower in implants placed in grafted bone sites when compared to nongrafted sites. Chiapasco et al studied this topic in a strategically important systematic review that addressed many issues concerning bone grafting for implants, including the resorption pattern of grafted bone, the timing of implant placement, the loading time of implant placement, and the survival and success rates of implants in grafted versus native bone.9 This review further considered the variety of graft materials and the effect of the type of materials used on implant survival. The authors concluded that the capacity of bone grafts to maintain volume was variable and that the survival and success rates of implants placed in grafted bone sites were lower compared to implants placed in native bone, especially in cases of extensive reconstructions. Evidence supporting a staged versus combined grafting and implant placement approach was presented. Despite the reported high predictability, when considering bone grafting to form bone to enhance implant placement opportunities for increased dental implant length, limitations exist, including greater morbidity and related second-site morbidity, the possibility of additional surgical procedures, prolonged healing periods, additional risks associated with increased numbers of interventions, and increased costs.
While alveolar bone grafting procedures have reported high success, the observation of potentially lower implant success in grafted bone sites was noted early in the development of techniques to treat the resorbed maxilla. For example, implant failures were 11% versus 25% in native versus grafted bone10 and 19% and 40.6% in "non-compromised" and "compromised" sites, respectively.11 Increased failure of implants in grafted bone is also found in more recent publications, such as in a retrospective analysis of single-tooth implants by Sesma et al, which demonstrated the negative impact of bone grafting on implant survival.12 Of 988 implants, 121 were placed in grafted bone, and over a 6-year follow-up period the authors reported 8.8%, 7.3%, and 1.6% failure after sinus augmentation, in bone block grafted areas, and in native bone, respectively. In a retrospective study of 2,729 implants (953 in grafted bone) in 1,222 patients, the 5- and 10-year implant survival rates were 92% and 87% respectively for native bone and 90% and 79% respectively for grafted bone. The late loss of implants may reflect underlying metabolic factors influencing bone mass.13
A retrospective study of 774 implants in 202 patients used generalized estimating equation analysis to determine that bone grafting was a risk factor for implant failure (P = .054). Additionally, maxillary implants were found to be a risk factor for implant failure in this study.14 In a retrospective study involving autogenous alveolar block grafts, implant failure was associated with bone graft failure (hazard ratio [HR] = 16.47), bone graft volume (horizontal and vertical; HR = 2.49), and location (posterior > anterior; HR = 2.51).15 Two recent and large retrospective studies of 30,959 implants16 and 6,977 implants17 both concluded that bone grafting was a significant factor associated with increased implant failure. These large retrospective studies indicate that while high implant survival can be achieved, there is increased risk of implant failure in grafted bone.
In addition to the moderate increased risks of implant failure in grafted bone, the issue of surgical complications must be acknowledged. Large block grafts appear to be associated with complications, including dehiscence.15 In one study, approximately one-third of grafts demonstrated partial or complete dehiscence.18 Complications delay treatment and increase treatment cost. Additionally, complications associated with reduced regenerated bone volume may negatively influence subsequent steps and planned therapy and require alternative plans, more complex prostheses, and additional surgical interventions. While in many patient cases bone grafting is necessary to advance implant therapy, the limitations and complications associated with bone grafting procedures have led to a search for nongrafted solutions.
Nongrafted Solutions
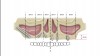
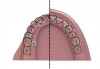
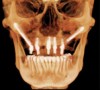
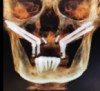
Today, at least four readily accessible nongrafting solutions are available for the provision of dental implant therapy to patients who present with alveolar bone volumes that preclude the use of standard-dimension implants and the conventional number of implants. These include tilted implants (eg, All-on-4),19 pterygoid implants and trans-sinus implants,20 zygomatic implants,21 and short implants,22 all of which allow for a reduced number of implants to be used. Graftless solutions for severe and moderate alveolar resorption of the edentulous maxilla are illustrated in Figure 1 and Figure 2.23,24
For the purpose of this article, narrow implants (≤3 mm) have been excluded as a nongrafting alternative because of the reported high success of horizontal bone regeneration that often averts narrow implant use. Given their potential biomechanical limitations, the use of narrow implants may be restricted for reduced mesiodistal dimension in single-tooth applications.
Tilted Implants
The use of implants in non-axial orientations was a latter development in the advancement of implant-supported fixed prostheses strategies. The pioneering observations of Krekmanov et al in 2000, presented in clinical study and theoretical considerations,23 set the stage for a transformation in implant treatment planning and therapy. In treatment of edentulous patients with maxillary and mandibular implant-supported fixed prostheses, 4-year cumulative survival rates of 95.7% for tilted implants and 92.5% for non-tilted implants were reported. Additionally, from a patient-based model it was calculated that tilting the implant actually reduced the damaging forces acting upon the implants three-fold. The authors correctly envisioned that tilted implants would enable effective use of dental implants without the need for auxiliary inferior alveolar nerve transposition or maxillary bone grafting.
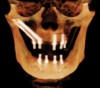
Building on this concept, Maló and co-workers advanced the broad use of tilted implants for restoration of the edentulous mandible and maxilla. In the maxilla, these implants may be used to preclude the alternative of sinus grafting (Figure 3 and Figure 4). The authors indicated that their intent was to "develop and document a simple, safe, and effective surgical and prosthetic protocol for immediate function (within 2 hours) of four implants supporting a fixed prosthesis."19 This first study in the edentulous mandible recorded a cumulative implant survival rate of 95.2%, which is comparable to conventional therapies.
Maló et al subsequently challenged maxillary edentulism using tilted distal implants, invoking improved implant anchorage through engagement of the cortical bone of the wall of the sinus as well as the nasal fossa, thus circumventing the need for grafting.25 In the retrospective study of 128 implants in 32 patients where implants were immediately loaded with all-acrylic prostheses, a 1-year implant survival rate of 97.6% was achieved. In a recent 10- to 18-year longitudinal study of 471 maxillary "All-on-4" patients involving 1,884 implants, the implant cumulative survival and success rates were 93% and 91.7%, respectively. The incidence of biologic and mechanical complications was 11.8% and 36.7%, respectively.26 There were reported complications, yet these early studies indicated that this approach offered new opportunities to treat the resorbed edentulous maxilla with high predictability.
The use of only four implants in a tilted arrangement has been debated. A recent review that considered the impact of the number of implants on therapeutic success after 5 or more years revealed no statistical difference between the axial and tilted implant therapeutic concepts.27 This study failed to demonstrate any effect of implant number on the success of implant-supported complete prostheses at the level of implant survival, marginal bone loss, prosthesis survival, or prosthesis complications.
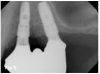
Titled implants are commonly used when there is insufficient native bone volume in zone 3 of the edentulous maxilla.24 As an example, Figure 5 shows a preoperative radiograph indicating the pneumatization of the sinuses with sufficient bone volume in zone 2 to accommodate distally tilted implants. The postoperative radiograph (Figure 6) reveals the position of the tilted implants and the resulting prosthesis. An additional benefit of this technique is an increased anterior-posterior spread of the abutments.
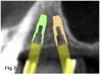
This approach has become more prevalent over the past 15 years, and its use has been extended beyond treatment of the severely resorbed maxilla. Clinicians have adopted the use of tilted implants to maximize the utilization of existing bone and avoid grafting procedures, often using only four implants distributed in an anterior-posterior direction. The tilting of distal implants to avoid sinus grafting can also be applied to unilateral fixed dental prostheses (Figure 7 through Figure 9).
In support of using tilted implants, a systematic review involving both maxillary and mandibular implant prostheses (N = 1,201) and 4,804 implants recorded a 3-year cumulative survival rate of 99%.28 A more recent systematic review as well as a meta-analysis reiterated the conclusion that distally tilted implants were not at greater risk for failure or marginal bone loss than axially placed implants.29,30
Zygomatic Implants
Brånemark and colleagues in the late 1980s introduced the zygomatic implant as a solution for the severely resorbed maxilla. Brånemark et al later described the treatment of 28 patients with severely resorbed edentulous maxillae using 52 zygomatic and 106 conventional implants. After a minimum of 5 to 10 years of follow-up they reported minimal complications and three out of 52 zygomatic fixture failures. They concluded that this style implant provided an additional approach to treatment of the severely resorbed edentulous maxilla.21
In the past nearly two decades, the use of zygomatic implants has expanded via alternative (extrasinus) methods,31 the introduction of the quad-zygoma implant treatment,32 and unilateral zygoma implant therapies.33 When comparing the use of zygomatic implants in patients in whom sinus grafting and conventional implants were indicated, Bedrossian et al suggested that patients treated with the zygomatic implants benefited from a less invasive procedure and immediate rehabilitation following a single surgery.34 Zygomatic implants can be used to provide for immediate functional rehabilitation in diverse clinical situations (Figure 10 through Figure 12).
Several systematic reviews have indicated high cumulative survival rates for zygomatic implants after 5 to 12 years.35,36 Regarding the timing of loading, immediate loading may be preferred over a delayed approach as indicated by statistically higher implant survival rates of immediately loaded zygomatic implants. This may be related to the relative absence of alveolar stability following placement and healing of some zygomatic implants.
Reported possible limitations associated with zygomatic implants include complex surgical placement that requires special training and often involves general anesthesia; a potentially complicated restorative phase of treatment, which demands resolution of palatally located abutments; significant incidence of sinusitis; and possible soft-tissue complications around abutments.35,37 Facial paresthesia and intraoral and extraoral fistulas also have been reported.37 Interestingly, Aparicio et al recently offered a novel set of four criteria ("ORIS") for measuring zygomatic implant therapeutic success that accounts for what may have been considered common complications38: O = offset measurement relative to prosthetic (tooth) positioning; R = rhino-sinus status based on radiographic and clinical findings; I = inflammation/infection of soft tissue; and S = stability of implant that allows limited mobility until dis-osseointegration occurs. Most concerning is that the possible eventual failure of a zygomatic implant requires complicated treatment compared with conventional implant failure.35
Therapeutic innovations such as placement of the implant in a more lateral position have been used to overcome the palatal location of the abutment and reduce reported development of sinusitis.36,39 Reported prosthetic complications include prosthetic and abutment screw loosening, framework fracture, and abutment tooth wear, paralleling the experiences of conventional implant acrylic/metal hybrid prostheses.31 Improvement in fabrication techniques offered through the use of digital technology may reduce these prosthetic complications.
The aggregate data from multiple prospective studies, retrospective studies, and systematic reviews demonstrate a high success rate for zygomatic implants, albeit with surgical complications that are unique to the implant as well as common prosthetic problems. Given their unique design and location of placement, zygomatic implants are associated with such reported surgical complications as sinusitis, fistulas, paresthesia, extraoral bruising, and labial lacerations.40 The use of zygomatic implants for rehabilitation of the severely resorbed maxilla, traumatized maxilla, and therapeutically resected maxilla provides a unique approach to dental and orofacial rehabilitation using dental implants in an area that would be otherwise inaccessible. This approach, when compared with grafting solutions, is direct and efficient.41
Pterygoid Implants
An early attempt to utilize existing bone of the maxilla to gain support for dental implants and improve function involved the use of bone of the maxillary tuberosity and pterygoid region. Tulasne in 1989 proposed the idea of using the relatively dense bone at the interface of the maxillary tuberosity, the palatine bone, and the pterygoid process of the sphenoid bone.42 Implants are often tilted in an acute angle with respect to the occlusal plane. Angles have been reported to be between 45 degrees and 75 degrees.43
Balshi et al recognized the many anatomic constraints posed by the edentulous maxilla can create challenges to implant placement with or without grafting.44 They suggested that implants engaging the pterygomaxillary plate for stability would successfully integrate to provide support for implant prostheses and, importantly, eliminate posterior cantilevers. Graves further advocated the use of such implants for overcoming anatomical limitations when placing implants in the edentulous maxilla.45
In a preliminary study involving 44 patients and 187 implants (51 pterygoid implants), a success rate of 86.3% for the pterygoid implants was recorded.44 In a subsequent publication reporting on 1,817 implants (356 pterygoid implants) in 189 subjects, 11.5% of pterygoid implants failed at second-stage surgery and only one other implant failed over the 6-month to 9-year follow-up period, resulting in an 88.2% survival rate after an average of 4.68 years of function. Among the limitations of therapy identified in these reports was the limited oral hygiene access associated with the distal aspect of the prosthesis.46
These early studies involved machined-surface implants. A recent systematic review of pterygoid implants indicated that studies after 2005 involving moderately rough surface implants showed much higher implant survival data. Araújo et al observed that in studies published after 2007 the pterygoid implant survival rate was 96.24%.43 The 10-year survival rate was 94.85%. The authors also addressed the unique surgical requirements for this technique, which involved a significant learning curve, and indicated that the procedure is associated with lower costs, shorter healing times, and less overall morbidity when compared with grafting approaches for treatment of the resorbed posterior maxilla. While a somewhat infrequently used surgical approach, the use of pterygoid implants to enable the avoidance of grafting of the edentulous posterior maxilla offers clinicians an additional treatment method that is supported by modest clinical evidence.
Short Implants
Many researchers have defined "short" implants in different ways. However, the existence of 6 mm and 7 mm implants for several decades would suggest that implants longer than 8 mm should not be considered short implants.2 Also, implants ≤6 mm have been referred to as "ultra-short" implants, but in this article the term "short" implant refers to an implant ≤6 mm in length.47
Studies have demonstrated that implants of 4 mm to 6 mm in length can be used for rehabilitations that otherwise would involve bone grafting. Even multiple splinted implants of 4 mm in length were shown to provide 92.2% implant survival after 5 years of function in the resorbed posterior mandible.48 Similarly, in a 5-year study of implant treatment of posterior partial edentulism, Felice et al demonstrated that 5 mm length implants achieved similar results to conventional implants in augmented bone.49 Other studies showed that 6 mm height x 4 mm diameter implants provided high (>95%) cumulative survival rates over 5 years.50-53 However, another study reported reduced single-tooth implant survival for 6 mm long implants versus longer implants.54 One possible explanation for the reduced survival may be the different implant design (one-piece) used in the investigation; another explanation could be related to the biomechanics of a single-tooth implant versus splinted implants.
Additional studies of single-tooth 6 mm implants did not report lower survival rates for short versus conventional-length implants.51,55 Short implants may be used for single-tooth, fixed dental prostheses and implant-supported fixed prostheses to allow bone augmentation to be avoided in cases where insufficient bone height will not permit the use of conventional implants.
One reason for the success of short implants initially after placement may be because, despite their reduced length, they can achieve sufficient primary stability and develop high secondary stability.56 In addition, recent systematic reviews demonstrate that marginal bone loss at short implants is significantly less than that at conventional, longer implants.57
Several systematic reviews support the use of short dental implants.22,58 Ravida et al indicated differential implant 5-year survival rates in the maxilla (90%) and mandible (96%) and minimal bone loss of 0.53 mm.59 While the most common prosthetic complication was screw loosening, splinted implants displayed reduced screw loosening (relative risk [RR] = 15.2) and reduced implant failure (RR = 1.96). Papaspyridakos et al reported that the 1- to 5-year survival rates for short implants ranged from 86.7% to 100% and survival rates of standard implants ranged from 95% to 100% (RR = 1.29).60 They recorded higher prosthetic complication rates for short implants versus standard implants and concluded that shorter implants should be selected carefully due to the lower survival rates and greater prosthetic complications. In contrast, Atieh et al investigated short implants for treatment of posterior partial edentulism and concluded that, despite the low numbers of representative implants in longer-term studies, short implants were an acceptable alternative to longer implants placed in grafted bone.61 Lin et al considered short implant survival in a large retrospective study of dental implant therapy and concluded that implant length was not a factor that influenced short-term implant survival, but short implants were a risk factor for long-term implant survival.16 The longer-term outcome of short implants must be considered underdefined at present.59
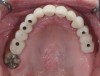
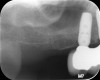
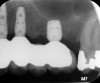
In a recent systematic review of short implants for the treatment of the posterior maxilla and mandible that included 13 randomized clinical trials, no differences were found in implant survival at 1, 3, 5, and 10 years.57 The authors concluded that short dental implants presented fewer postoperative complications compared with augmentation procedures, less marginal bone loss than conventional-length implants, and no significant difference in implant failure versus conventional-length implants.57 Short implants can be utilized to avoid grafting in strategic locations, particularly inferior to a modestly pneumatized maxillary sinus (Figure 13 through Figure 15).
Many clinical reports and systematic reviews have repeatedly indicated relative advantages of short implants compared to alveolar augmentation or sinus grafting, such as reduced treatment time, fewer procedures, and lower complication rates. For example, Thoma et al stated that patients demonstrated a preference for short implants regarding short-term patient morbidity, treatment time, and price.62 When weighing the potential risks versus benefits of the use of short dental implants, this modality may be considered as an alternative treatment for the atrophic maxilla.
Guidelines for Use of Short Dental Implants
Based on published information regarding clinical outcomes of short dental implants compared to either conventional-length implants or bone augmentation procedures with the placement of conventional-length implants, several guidelines may be suggested. First, the use of short implants in the context of alveolar ridge resorption infers that larger crown-to-implant (C/I) ratios will be encountered. Hingsammer et al determined that a C/I ratio of greater than 1.7 for splinted 6.5 mm implants was associated with increased initial marginal bone loss.63 In a comparative clinical study involving 4 mm diameter x 6 mm length implants versus conventional-length implants, the short implant C/I ratio (1.7) and conventional implant C/I ratio (1.0) was not associated with marginal bone loss or implant complications.64 A small study of implants of <8 mm length statistically compared implants with C/I ratios of <2 to implants with C/I ratios of >2. The authors demonstrated similar implant survival and marginal bone loss.65
Another 1-year report of 6 mm implants where the average C/I ratio was 2.14 found that the high C/I ratio did not increase marginal bone loss or prosthetic complications.66 A similar conclusion was made regarding comparison of 6 mm versus 9-12 mm implants.67 While other studies indicate that C/I ratios of 2 to 3 are considered not to be a risk for implant failure, applied occlusal loads need to be carefully controlled. This is achieved by locating the long axis of the implant centrally through the occlusal table at the time of implant placement and controlling non-axial forces that markedly increase lateral loads.68
When using multiple implants, short implants must be splinted to reduce potential prosthetic complications.59,69 Most studies of short implants indicate that minimal marginal bone loss occurs following placement and function. Implants should be placed in bone of sufficient quality and quantity (>1 mm bone circumferentially) with proper mucosal tissue thickness (>2 mm) and abutment length (>2 mm) to minimize marginal bone changes. Reductions of 1 mm of interfacial bone support can markedly alter the interfacial shear strength of the interface, which can lead to overload and possible implant loss.
Little data exists that discusses the impact of immediate versus one-stage versus two-stage surgery on the survival of short dental implants. A comparison of immediate loading of 6 mm versus 10 mm molar implants placed in bound edentulous spaces indicated that immediate loading associated with high implant stability quotient (ISQ) values may be possible.70 The splinting of 6.5 mm implants and immediate loading has been reported to provide 100% short-term success.71 The impact of immediate loading of unsplinted implants in unbound spaces, terminal abutments, or full-arch restorations remains largely undefined. Currently, the present authors' experience has generally involved a two-stage approach despite the high primary stability achievable in placing short dental implants. While this may be less significant in cross-arch splinted patient cases,72 relatively large C/I ratios associated with short implants may increase stresses at the healing bone-to-implant interfaces during immediate load function or provisional restorative function.
Longer-term data for short implants is scarce. Regarding longevity, short implants should be carefully considered for patients with risk factors associated with greater chances of long-term implant failure. Such patients may include those with advanced periodontitis as a cause of tooth loss, possibly those with diabetes, smokers, proton pump inhibitor users, selective seratonin reuptake inhibitor users, and those with bruxism. In healthy patients with reduced bone volume, well-placed and carefully restored short implants offer an alternative to grafting solutions that may be needed to accommodate longer implants.
An overview of the nongrafting solutions available for the provision of implant therapy discussed in this article is provided in Table 1, "Nongrafting Dental Implant Solutions," which may be viewed online at compendiumce.com/go/2014.
Summary
Advances in implant design and therapeutics over the past few decades have challenged the traditional concepts of implant anchorage in residual alveolar ridges, the notion of axial (parallel) biomechanics, and the process of osseointegration. The use of pterygoid, zygomatic, tilted, and short implants has provided novel ways of achieving osseointegration of implants to support dental prostheses. Studies and systematic reviews now exist that cite a high degree of success for each of these treatment modalities, although these implants all have associated limitations and unique complications that must be addressed at the time of planning and placement to limit potential risks. Because these nongrafting solutions, however, circumvent local- and second-site morbidity, reduce treatment time, and could be aligned with immediate loading protocols, they offer selected benefits for implant rehabilitation.
About the Authors
Lyndon F. Cooper, DDS, PhD
Associate Dean for Research, Head, Department of Oral Biology, University of Illinois at Chicago, Chicago, Illinois; Diplomate, American Board of Prosthodontics
Ghadeer Thalji, DDS, PhD
Co-director, Graduate Prosthodontics, Associate Professor, University of Illinois at Chicago, Chicago, Illinois; Fellow, American College of Prosthodontists
Sandra Al-Tarawneh, DDS, MS
Visiting Clinical Associate Professor, University of Illinois at Chicago, Chicago, Illinois; Fellow, American College of Prosthodontists
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387-416.
2. Friberg B, Gröndahl K, Lekholm U, Brånemark PI. Long-term follow-up of severely atrophic edentulous mandibles reconstructed with short Brånemark implants. Clin Implant Dent Relat Res. 2000;2(4):
184-189.
3. Lekholm U, Zarb GA. Tissue integrated prostheses: osseointegration in clinical dentistry. In: Brånemark PI, Zarb GA, Albrektsson T, eds. Patient Selection and Preparation. Chicago, IL: Quintessence Publishing; 1985:199-209.
4. Kahnberg KE, Nilsson P, Rasmusson L. Le Fort I osteotomy with interpositional bone grafts and implants for rehabilitation of the severely resorbed maxilla: a 2-stage procedure. Int J Oral Maxillofac Implants. 1999;14(4):571-578.
5. Duttenhoefer F, Souren C, Menne D, et al. Long-term survival of dental implants placed in the grafted maxillary sinus: systematic review and meta-analysis of treatment modalities. PLoS One. 2013;8(9):e75357.
6. Esposito M, Grusovin MG, Felice P, et al. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2009;2009
(4):CD003607.
7. Shan XF, Chen HM, Liang J, et al. Surgical reconstruction of maxillary and mandibular defects using a printed titanium mesh. J Oral Maxillofac Surg. 2015;73(7):1437.e1-1437.
8. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(suppl):49-70.
9. Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Impl Res. 2006;17(suppl 2):136-159.
10. Lekholm U, Wannfors K, Isaksson S, Adielsson B. Oral implants in combination with bone grafts. A 3-year retrospective multicenter study using the Brånemark implant system. Int J Oral Maxillofac Surg. 1999;28
(3):181-187.
11. Snauwaert K, Duyck J, van Steenberghe D, et al. Time dependent failure rate and marginal bone loss of implant supported prostheses: a 15-year follow-up study. Clin Oral Investig. 2000;4(1):13-20.
12. Sesma N, Pannuti C, Cardaropoli G. Retrospective clinical study of 988 dual acid-etched implants placed in grafted and native bone for single-tooth replacement. Int J Oral Maxillofac Implants. 2012;27(5):1243-1248.
13. Tran DT, Gay IC, Diaz-Rodriguez J, et al. Survival of dental implants placed in grafted and nongrafted bone: a retrospective study in a university setting. Int J Oral Maxillofac Implants. 2016;31(2):310-317.
14. Borba M, Deluiz D, Lourenço EJV, et al. Risk factors for implant failure: a retrospective study in an educational institution using GEE analyses. Braz Oral Res. 2017;31:e69.
15. Schwartz-Arad D, Ofec R, Eliyahu G, et al. Long term follow-up of dental implants placed in autologous onlay bone graft. Clin Implant Dent Relat Res. 2016;18(3):449-461.
16. Lin G, Ye S, Liu F, He F. A retrospective study of 30,959 implants: risk factors associated with early and late implant loss. J Clin Periodontol. 2018;45(6):733-743.
17. Zhou N, Dong H, Zhu Y, et al. Analysis of implant loss risk factors especially in maxillary molar location: a retrospective study of 6977 implants in Chinese individuals. Clin Implant Dent Relat Res. 2019;21(1):138-144.
18. Tosun E, Akkocaoğlu M, Tüz HH, et al. Complications associated with anterior iliac bone grafting for the reconstruction of dentoalveolar defects. J Craniofac Surg. 2019;30(4):980-984.
19. Maló P, Rangert B, Nobre M. "All-on-Four" immediate-function concept with Brånemark System implants for completely edentulous mandibles: a retrospective clinical study. Clin Implant Dent Relat Res. 2003;5(suppl 1):2-9.
20. Jensen OT, Cottam J, Ringeman J, Adams M. Trans-sinus dental implants, bone morphogenetic protein 2, and immediate function for all-on-4 treatment of severe maxillary atrophy. J Oral Maxillofac Surg. 2012;70(1):141-148.
21. Brånemark P-I, Gröndahl K, Öhrnell L-O, et al. Zygoma fixture in the management of advanced atrophy of the maxilla: technique and long-term results. Scand J Plast Reconstr Surg Hand Surg.2004;38(2):70-85.
22. Annibali S, Cristalli MP, Dell'Aquila D, et al. Short dental implants: a systematic review. J Dent Res. 2012;91(1):25-32.
23. Krekmanov L, Kahn M, Rangert B, Lindström H. Tilting of posterior mandibular and maxillary implants for improved prosthesis support. Int J Oral Maxillofac Implants. 2000;15(3):405-414.
24. Bedrossian E, Bedrossian EA. Systematic treatment planning protocol of the edentulous maxilla for an implant-supported fixed prosthesis. Compend Contin Educ Dent. 2019;40(1):20-25.
25. Maló P, Rangert B, Nobre M. All-on-4 immediate-function concept with Brånemark System implants for completely edentulous maxillae: a 1-year retrospective clinical study. Clin Implant Dent Relat Res. 2005;7(suppl 1):S88-S94.
26. Maló P, de Araújo Nobre M, Lopes A, et al. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin Implant Dent Relat Res. 2019;21(4):565-577.
27. de Luna Gomes JM, Lemos CAA, Santiago Junior JF, et al. Optimal number of implants for complete-arch implant-supported prostheses with a follow-up of at least 5 years: a systematic review and meta-analysis. J Prosthet Dent. 2019;121(5):766-774.
28. Patzelt SB, Bahat O, Reynolds MA, Strub JR. The all-on-four treatment concept: a systematic review. Clin Implant Dent Relat Res. 2014;16(6):836-855.
29. Lin WS, Eckert SE. Clinical performance of intentionally tilted implants versus axially positioned implants: a systematic review. Clin Oral Implants Res. 2018;29(suppl 16):78-105.
30. Chrcanovic BR, Albrektsson T, Wennerberg A. Tilted versus axially placed dental implants: a meta-analysis. J Dent. 2015;43(2):149-170.
31. Maló P, de Araújo Nobre M, Lopes A, et al. Extramaxillary surgical technique; clinical outcome of 32 patients rehabilitated with747 zygomatic implants with a follow-up between six months and 7 years. Clin Implant Dent Relat Res. 2015;17(suppl 1):e153-e162.
32. Davó R, David L. Quad zygoma: technique and realities. Oral Maxillofac Surg Clin North Am. 2019;31(2):285-297.
33. Higuchi KW. The zygomaticus fixture: an alternative approach for implant anchorage in the posterior maxilla. An R Australas Coll Dent Surg. 2000;15:28-33.
34. Bedrossian E, Rangert B, Stumpel L, Indresano T. Immediate function with the zygomatic implant: a graftless solution for the patient with mild to advanced atrophy of the maxilla. Int J Oral Maxillofac Implants. 2006;21(6):937-942.
35. Chrcanovic BR, Albrektsson T, Wennerberg A. Survival and complications of zygomatic implants: an updated systematic review. J Oral Maxillofac Surg. 2016;74(10):1949-1964.
36. Tuminelli FJ, Walter LR, Neugarten J, Bedrossian E. Immediate loading of zygomatic implants: a systematic review of implant survival, prosthesis survival and potential complications. Eur J Oral Implantol. 2017;10(supp 1):79-87.
37. Aparicio C, Manresa C, Francisco K, et al. Zygomatic implants: indications, techniques and outcomes, and the zygomatic success code. Periodontol 2000. 2014;66(1):41-58.
38. Aparicio C, López-Piriz R, Albrektsson T. ORIS criteria of success for the zygoma-related rehabilitation: the (revisited) zygoma success code. Int J Oral Maxillofac Implants. 2020;35(2):366-378.
39. Aleksandrowicz P, Kusa-Podkańska M, Tomkiewicz W, et al. Platform switch hybrid zygoma implants improve prosthetics and marginal bone protection after extra-sinus placement. Clin Implant Dent Relat Res. 2020;22(2):186-192.
40. Molinero-Mourelle P, Baca-Gonzalez L, Gao B, et al. Surgical complications in zygomatic implants: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(6):e751-e757.
41. Davó R, Felice P, Pistilli R, et al. Immediately loaded zygomatic implants vs conventional dental implants in augmented atrophic maxillae: 1-year post-loading results from a multicentre randomised controlled trial. Eur J Oral Implantol. 2018;11(2):145-161.
42. Tulasne JF. Implant treatment of missing posterior dentition. In: Albrektson T, Zarb G, eds. The Brånemark Osseointegrated Implant. Chicago, IL: Quintessence Publishing; 1989:103-115.
43. Araújo RZ, Santiago Júnior JF, Cardoso CL, et al. Clinical outcomes of pterygoid implants: Systematic review and meta-analysis. J Craniomaxillofac Surg. 2019;47(4):651-660.
44. Balshi TJ, Lee HY, Hernandez RE. The use of pterygomaxillary implants in the partially edentulous patient: a preliminary report. Int J Oral Maxillofac Implants. 1995;10(1):89-98.
45. Graves SL. The pterygoid plate implant: a solution for restoring the posterior maxilla. Int J Periodontics Restorative Dent. 1994;14(6):512-523.
46. Balshi TJ, Wolfinger GJ, Balshi SF 2nd. An analysis of 356 pterygomaxillary implants in edentulous arches. Int J Oral Maxillofac Implants. 1999;14(3):398-406.
47. Neldam CA, Pinholt EM. State of the art of short dental implants: a systematic review of the literature. Clin Implant Dent Relat Res. 2012;14(4):
622-632.
48. Slotte C, Grønningsaeter A, Halmøy AM, et al. Four-millimeter-long posterior-mandible implants: 5-year outcomes of a prospective multicenter study. Clin Implant Dent Relat Res. 2015;17(suppl 2):
e385-e395.
49. Felice P, Barausse C, Pistilli R, et al. Five-year results from a randomised controlled trial comparing prostheses supported by 5-mm long implants or by longer implants in augmented bone in posterior atrophic edentulous jaws. Int J Oral Implantol (New Malden). 2019;
12(1):25-37.
50. Zadeh HH, Guljé F, Palmer PJ, et al. Marginal bone level and survival of short and standard-length implants after 3 years: an open multi-center randomized controlled clinical trial. Clin Oral Implants Res. 2018;29(8):894-906.
51. Thoma DS, Haas R, Sporniak-Tutak K, et al. Randomized controlled multicentre study comparing short dental implants (6 mm) versus longer dental implants (11-15 mm) in combination with sinus floor elevation procedures: 5-year data. J Clin Periodontol. 2018;45(12):1465-1474.
52. Felice P, Barausse C, Pistilli R, et al. Short implants versus longer implants in vertically augmented posterior mandibles: result at 8 years after loading from a randomised controlled trial. Eur J Oral Implantol. 2018;11(4):385-395.
53. Bechara S, Kubilius R, Veronesi G, et al. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10-mm) dental implants: a randomized controlled trial with a 3-year follow-up. Clin Oral Implants Res. 2017;28(9):1097-1107.
54. Naenni N, Sahrmann P, Schmidlin PR, et al. Five-year survival of short single-tooth implants (6 mm): a randomized controlled clinical trial. J Dent Res. 2018;97(8):887-892.
55. Guljé FL, Raghoebar GM, Vissink A, Meijer HJA. Single crowns in the resorbed posterior maxilla supported by either 11-mm implants combined with sinus floor elevation or 6-mm implants: a 5-year randomised controlled trial. Int J Oral Implantol (New Malden). 2019;12(3):315-326.
56. Alonso FR, Triches DF, Mezzomo LAM, et al. Primary and secondary stability of single short implants. J Craniofac Surg. 2018;29(6):e548-e551.
57. Altaib FH, Alqutaibi AY, Al-Fahd A, Eid S. Short dental implant as alternative to long implant with bone augmentation of the atrophic posterior ridge: a systematic review and meta-analysis of RCTs. Quintessence Int. 2019;50(8):636-650.
58. Srinivasan M, Vazquez L, Rieder P, et al. Survival rates of short (6 mm) micro-rough surface implants: a review of literature and meta-analysis. Clin Oral Implants Res. 2014;25(5):539-545.
59. Ravidà A, Barootchi S, Askar H, et al. Long-term effectiveness of extra-short (≤6 mm) dental implants: a systematic review. Int J Oral Maxillofac Implants. 2019;34(1):68-84.
60. Papaspyridakos P, De Souza A, Vazouras K, et al. Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: a meta-analysis. Clin Oral Implants Res. 2018;29(suppl 16):8-20.
61. Atieh MA, Zadeh H, Stanford CM, Cooper LF. Survival of short dental implants for treatment of posterior partial edentulism: a systematic review. Int J Oral Maxillofac Implants. 2012;27(6):1323-1331.
62. Thoma DS, Haas R, Tutak M, et al. Randomized controlled multicentre study comparing short dental implants (6 mm) versus longer dental implants (11-15 mm) in combination with sinus floor elevation procedures. Part 1: demographics and patient-reported outcomes at 1 year of loading. J Clin Periodontol. 2015;42(1):72-80.
63. Hingsammer L, Watzek G, Pommer B. The influence of crown-to-implant ratio on marginal bone levels around splinted short dental implants: a radiological and clinical short term analysis. Clin Implant Dent Relat Res. 2017;19(6):1090-1098.
64. Hadzik J, Krawiec M, Sławecki K, et al. The influence of the crown-implant ratio on the crestal bone level and implant secondary stability: 36-month clinical study. Biomed Res Int. 2018;2018:4246874.
65. Ghariani L, Segaan L, Rayyan MM, et al. Does crown/implant ratio influence the survival and marginal bone level of short single implants in the mandibular molar? A preliminary investigation consisting of 12 patients. J Oral Rehabil. 2016;43(2):127-135.
66. Guljé FL, Raghoebar GM, Erkens WA, Meijer HJ. Impact of crown-implant ratio of single restorations supported by 6-mm implants: a short-term case series study. Int J Oral Maxillofac Implants. 2016;31(3):672-675.
67. Guarnieri R, Di Nardo D, Gaimari G, et al. Short vs. standard laser-microgrooved implants supporting single and splinted crowns: a prospective study with 3 years follow-up. J Prosthodont. 2019;28(2):e771-e779.
68. Verri FR, Santiago Junior JF, de Faria Almeida DA, et al. Biomechanical influence of crown-to-implant ratio on stress distribution over internal hexagon short implants: 3-D finite element analysis with statistical test. J Biomech. 2015;48(1):138-145.
69. Toniollo MB, Macedo AP, Pupim D, et al. Finite element analysis of bone stress in the posterior mandible using regular and short implants, in the same context, with splinted and nonsplinted prostheses. Int J Oral Maxillofac Implants. 2017;32(4):e199-e206.
70. Weerapong K, Sirimongkolwattana S, Sastraruji T, Khongkhunthian P. Comparative study of immediate loading on short dental implants and conventional dental implants in the posterior mandible: A randomized clinical trial. Int J Oral Maxillofac Implants. 2019;34(1):141-149.
71. Anitua E, Flores C, Flores J, Alkhraisat MH. Clinical effectiveness of 6.5-mm-long implants to support two-implant fixed prostheses in premolar-molar region: the influence of immediate loading and the length of splinting implant. J Prosthodont. 2019;28(2):e688-e693.
72. Maló P, de Araújo Nobre MA, Lopes AV, Rodrigues R. Immediate loading short implants inserted on low bone quantity for the rehabilitation of the edentulous maxilla using an All-on-4 design. J Oral Rehabil. 2015;42(8):615-623.