You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Four key breakthroughs have driven the evolution of dental implantology as it is known today: (1) the discovery of osseointegration by Dr. P-I Branemark1; (2) the application of computed tomography (CT) imaging technology for "optimal, prosthetically directed implant placement"2-4; (3) computer-generated stereolithographic surgical guides5-8; and (4) cone-beam CT (CBCT), which reduced radiation exposure and improved access for the private practice sector.9,10
In the late 1980s, 3-dimensional (3D) imaging using medical-grade, spiral CT became an important yet controversial tool for implant dentistry. Three-dimensional imaging allowed accurate diagnosis of regional anatomy and more personalized planning for surgery via planning software that enabled improved surgical-prosthetic collaboration. However, no methodology was available to transfer and apply the computer-based surgical plan directly to the operating field.
Guided Surgery Background
In 2000, CBCT became available and allowed for significantly less radiation exposure.9,10 In 2002, the ability to generate stereolithographic surgical guides from a CBCT-based computer software plan negated much of the radiation exposure concerns related to effective-dose safety for "elective" therapies.11 Today, an emerging standard of care is the use of cross-sectional CBCT imaging for planning and a stereolithographic surgical guide when executing surgery.12,13
At their inception, stereolithographic surgical guides were sequential drilling templates that allowed osteotomy sites to be enlarged and were used to control the buccolingual and mesiodistal planes of space.14 This application has been referred to as "partially guided" implant surgery. The effect of stereolithographic surgical guides was significant, improving entry point deviations and angle discrepancies by nearly 50% when compared to conventional freehand surgery.15 However, it was the production of fully guided implant surgery that improved accuracy to submillimeter levels and enabled control in all three planes of space: buccolingual, mesiodistal, and apicocoronal.16 Rotational timing is also possible with fully guided systems, though this is implant- and/or guide-manufacturer dependent.8,17
Today, the advent of intraoral surface scanners and computer-aided design/computer-aided milling (CAD/CAM) has made guided, full-arch, immediate-function treatment for the edentulous and terminally dentate patient simpler and more predictable.18 Computer-generated stereolithographic guides used for all modalities of partially or totally guided surgery are referred to as "static" guides, because once they are constructed there is no opportunity to change the treatment plan and remain "guided." Despite their improvements over previous methods, the use of stereolithographic guides can be hampered by inaccurate planning and fit, movement of the guide during the surgical operation, and bone density difficulties that may require angulation changes during placement.8 Unfortunately, inaccuracies in the guides typically are not discovered until the time of surgery or, worse, after the implants have been placed.
Dynamic navigation (or "virtual") surgery is relatively common in various areas of medicine such as craniomaxillofacial surgery, neurosurgery, and orthopedic/spine surgery.19,20 Surgical navigation systems are made possible by motion tracking technology, commonly referred to as a micron tracker camera.19,20 This technology is now also available in dentistry.
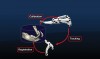
In dentistry, implant placement is possible using dynamic navigation because the micron tracker camera is able to relate the position of the patient's jaw to the position of the implant drill tip in real time. The relationship of the drill to the jaw is displayed instantaneously, allowing for continuous, immediate feedback on the mesiodistal, buccolingual, and apicocoronal positions of the implant drill (Figure 1). The three fundamental steps, which together enable the real-time mapping of the drill tip to the patient's planned CBCT image volume, are called registration, calibration, and tracking (Figure 2). The surgeon is thus able to prepare the osteotomy and place the implant based on the pre-planned, prosthetically directed implant position.
There are several advantages of this approach compared to the use of static guides: changes can be made during the surgery, the technology can be used with a standard surgical kit for any implant system, surgical navigation can be accomplished in 1 day (if circumstances permit), and the cost for constructing a static guide is eliminated. Further, when compared to freehand approaches, guidance enables a significant reduction in damage to soft tissue with a resultant decrease in infection risk, patient discomfort, and soft-tissue healing time. For dental implant surgery, dynamic navigation technology allows for real-time verification and validation of positional accuracy. The purpose of this article is to introduce readers to dynamic navigation and present a case using the technology.
Overview of Navigation System
Dynamic navigation systems track the position of the tip of the implant drill and map it to a pre-acquired CBCT scan of the patient's jaw to provide real-time drilling and placement guidance/feedback.21 When the drill approaches a pre-planned implant location, the system provides a "cross-hair" or "bulls-eye" display to help the surgeon navigate precisely to the planned drill entry point position, adjust the drill orientation to the planned angle, and navigate the osteotomy site along the planned trajectory to the planned depth.22 Once the osteotomy preparation is complete, the same approach can be used to guide the insertion of the implant itself.
The dynamic navigation system described here is the Navident system (ClaroNav, claronav.com). Other similar dynamic navigation systems include X-Guide Dynamic 3D Navigation (X-Nav, x-navtech.com), Image Guided Implant (IGI) Dentistry System (Image Navigation, image-navigation.com), YOMI® (Neocis, neocis.com), and Inliant® (Navigate Surgical, navigatesurgical.com). The Navident, X-Guide, IGI, and YOMI systems are US Food and Drug Administration approved. The Navident dynamic navigation system consists of five main components (Figure 1)2: (1) A notebook computer runs the system software and provides integrated planning and navigation functionalities. (2) A handpiece attachment consists of a universal handpiece-hugging adapter and an optically marked plastic component, referred to as the "drill tag." (3) A patient jaw attachment consists of a moldable thermoplastic stent that is situated directly on the patient's natural dentition (ie, the retainer) with a retainer arm extending from the retainer body. A fiducial marker attached to the retainer acts as a CT marker and allows for spatial registration of the patient relative to the jaw attachment. This same retainer is used during the implant surgery, thus maintaining the same relationship to the CBCT image that was obtained and used for case planning. (4) An optical positioning sensor (ie, micron tracker camera) detects the patterns printed on the handpiece and jaw attachments and constantly reports their relative positions to the dynamic system software. This allows the surgeon to intraoperatively reference and, in real time, verify and validate positional accuracy. (5) A compact, mobile cart (not shown in Figure 1) holds the laptop and positions the optical sensor above the patient.
Workflow: Stent, Scan, Plan, and Place
The navigation system described here has a digital workflow that involves four major steps:
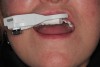
Stent-A thermoplastic retainer is molded over the dentition, left to harden, and then removed and trimmed to provide access to the intended implantation region. The retainer arm is made of the same thermoplastic material and includes a fix plate at its end, which is joined to the CT marker via a thumbscrew. The CT marker contains an aluminum fiduciary marker within it that can be identified in the CBCT. The fix plate is the common reference position between the CBCT scan and the surgery to which mathematical algorithms can be calculated so that spatial positioning is known to the system. When attached via the thumbscrew, the CT marker is rigidly locked with the retainer. The thermoplastic retainer and arm are attached via adhesive glue (Figure 3).
Scan-The CT marker, thermoplastic stent, and retainer arm with fix-plate apparatus are secured and seating accuracy is verified. Thereafter, a single jaw is scanned using a CBCT scanner (Figure 3).
Plan-The image data in Digital Imaging Communication in Medicine (DICOM) format is imported to the navigation system by a direct transfer using the local network. Registration of the fiducial image in the CT scan is performed (automatically) and verified by the user at this point. STL files of either the dentition or prosthetic wax-ups can be imported from optical scanning for prosthetically directed treatment planning (Figure 4). Virtual teeth are placed and adjusted to simulate the desired restoration, and supporting implants are placed in consideration of the prosthetic plan. While considerable time is spent in the planning phase, the surgeon has the opportunity to modify the plan during the operation if the surgical environment warrants change.
Place-The laptop displaying the plan is positioned over the patient. The customized thermoplastic stent is now attached to an optical marker tag (ie, jaw tag), which replaces the CT marker. The jaw tag is secured to the fix plate of the retainer arm with the use of a thumbscrew. The second optical marker component (ie, drill tag) is mounted to the handpiece, the drill's axis is calibrated, and then the first drill in the sequence is installed in the handpiece and its length (tip) is calibrated (Figure 5). Each drill tip for osteotomy site preparation is independently calibrated just prior to its use, followed by an accuracy check prior to drilling. Osteotomy site preparation is performed using the dynamic navigation technology. The motion of the handpiece is tracked against patient position. During drilling the operator can see axial, panoramic, and cross-sectional views in real time as the case progresses. A real-time "cross-hair" or "bulls-eye" target view provides distance in millimeters between the drill tip and the central axis, the angle of the drill in relation to the central length axis, and the distance (mm) between the tip of the drill and the apical end of the planned osteotomy (Figure 6).
While the surgeon is watching the computer monitor rather than focusing on patient anatomy, the surgical field is open for better treatment visibility compared to static guides. The drill tip calibration and accuracy checks are repeated after each drill size change. The surgeon conducts intrasurgical accuracy checks by touching rigid anatomical landmarks such as the surfaces of nearby teeth with the drill tip of osteotomy burs. If the indicated accuracy demonstrated by the software is insufficient, the surgeon can troubleshoot to find and eliminate the source of the inaccuracy by following a systematic procedure. If the issue cannot be resolved, the surgery can be aborted or the CBCT imaging and planning phases can be repeated. The advantage of this technology is the real-time verification and validation of implant position entry point, angulation, and depth, which can be ensured for instantaneous transparency (ie, surgical accountability).
Sources of Guidance Errors
Errors in the drill tip to CT image mapping may appear at any step in the workflow due to slight inaccuracies within the dynamic navigation system or associated with using the technology (eg, manufacturing tolerances, stent distortion, inaccurate calibration, patient motion during the CT scanning process, unstable seating of the jaw attachment, bending of arms or connectors during surgery). Operators must pay close attention throughout the process to minimize errors that could ultimately compromise accuracy.
Despite the navigation system's extraordinary detection accuracy, the surgeon's control (or ability to direct the handpiece) is inherently imperfect. Challenges with regard to his or her hand-eye coordination and personal fine motor control under stressful surgical conditions may influence individual results. Thus, operating with a freehand, standard implant handpiece without static guide control, as with the dynamic navigation system, does add an element of operator-dependent error.
Case Report
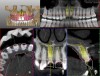
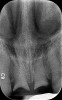
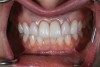
A 29-year-old Caucasian woman presented to the author's (GAM) practice for evaluation of teeth Nos. 8 and 9. The teeth were fractured at the free gingival margin and had sclerosed dental pulps (Figure 7 and Figure 8). The patient's medical history was significant for gastroesophageal reflux disorder (GERD), migraines, narcolepsy, attention deficit hyperactivity disorder (ADHD), and depression. She had no known drug allergies or drug idiosyncrasies and was determined to have an American Society of Anesthesiologists (ASA) II physical status.
A comprehensive periodontal examination was performed. A thermoplastic retainer and arm were fabricated for the patient with the fiduciary marker (CT marker) attached to the fix plate. Great care was taken regarding the fit of the thermoplastic stent to ensure proper seating. A CBCT scan (CS 9300, Carestream Dental, carestreamdental.com) of the maxilla was secured, and the DICOM data were registered into the planning software.
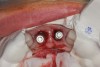
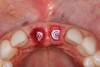
The surgery was performed under local-regional anesthesia. The thermoplastic retainer was placed over the remaining teeth and its fit/stability verified. Atraumatic extractions of teeth Nos. 8 and 9 were performed, and intact buccal bone was verified. Osteotomy site preparation and immediate implant placement were performed using the dynamic surgical navigation system. Two 3.6 mm x 9 mm implant fixtures (Astra Tech EV, Dentsply Sirona, dentsplysirona.com) were placed. SmartPegs (Osstell, osstell.com) were attached to the implants to show the trajectory of the fixture positioning. After implant placement, anorganic bovine bone matrix (Bio-Oss®, Geistlich Pharma, geistlich-na.com) was used to graft the implant alveolus "gap," and healing abutments were placed (Figure 9 and Figure 10). The patient was provided with an interim removable appliance for tooth replacement.
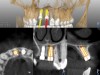
A post-placement CBCT scan was secured and compared to the preoperative CBCT plan using software inherent to the dynamic navigation system (Figure 11). Accuracy results from this case (preoperative plan compared to post-implant placement) were as follows: entry point deviation was 0.13 mm for tooth position No. 8 and 0.41 mm for No. 9; angle discrepancy was 4.3 degrees for No. 8 and 6.76 degrees for No. 9; implant apex depth deviation was 1.10 mm for No. 8 and 1.37 mm for No. 9.
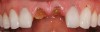
At 3 months, osseointegration was confirmed and screw-retained provisionals were used for soft-tissue grooming. Final prosthetic phase completion occurred at 6 months (Figure 12).
Discussion
Imaging technology has transformed the field of implant dentistry and has led to significant improvements in accuracy and greater predictability in prosthetic outcomes.23 A systematic review demonstrated that, on average, CT-guided implant surgery with static guides has around 1 mm entry point deviation and around 5 degrees of angle discrepancy when compared to treatment plans.23 However, that and another systematic review also demonstrated that large standard deviations exist (ranging up to 7.5 mm for entry point deviation and more than 15 degrees angle discrepancy).23,24 Static guided surgery has many challenges, most of which involve either planning errors or iatrogenic errors that occur during surgery. Certain clinical situations, such as limited mouth opening or interdental space limitations, may preclude the use of stereolithographic surgical guides. These types of limitations do not apply to dynamic navigation surgery.
Because static stereolithographic guides provide no reference of tooth position, most inaccuracies are realized after surgery. Dynamic navigation provides the ability to verify and validate, in real time, positional accuracy of osteotomy site preparation and implant placement. In addition, real-time navigation affords an opportunity to edit the plan during surgery. Because the operating field is fully visualized and unrestricted, changes to implant positioning or dimension can be implemented when regional anatomy warrants modification unforeseen during the planning phase. Improper implant placement has repeatedly been found to be a factor in esthetic failures and/or marginal bone loss.25,26
In the present case report, where the surgery was performed while the authors were still in a learning curve with dynamic navigation, the entry point deviations for tooth position Nos. 8 and 9 were 0.13 mm and 0.41 mm, respectively, and the respective angle discrepancies were 4.3 and 6.76 degrees. Block et al has demonstrated that dynamic navigation has the potential to further improve accuracy measurements compared to static guides but that, on average, the clinician must perform at least 20 cases using the technology before the learning curve is mastered.27 This is encouraging considering that other medical disciplines have reported a proficiency curve ranging from 60 to 500 cases depending on the surgical subspecialty.27,28 Like the introduction of guided surgery in 2002, accuracy and outcome success are largely proportional to planning and experience.
In a recent publication by Stefanelli et al, data were obtained on 231 implants placed in healed ridges using a flapless or minimal flap approach under dynamic guidance by a single surgeon using the same navigation system described here. Of the 89 arches operated on, 28 (125 implants) were fully edentulous. For all implants, the mean deviations (SD) were: 0.71 (0.40) mm for entry point (lateral) and 1 (0.49) mm at the apex (3D). The mean angle discrepancy was 2.26 degrees (1.62 degrees) from actual versus planned implant positions. The accuracy measurements for partially edentulous patients using a thermoplastic stent attachment and for fully edentulous patients using a mini-implant-based attachment were nearly identical. No significant accuracy differences were found between implant positions within the different sextants. Guided insertion of the implant itself reduced angular and apex location deviations. Most interestingly, the accuracy of implant placement improved during the study period, with the mean entry point, apex deviation, and overall angle discrepancy measured for the last 50 implants (0.59 mm, 0.85 mm, and 1.98 degrees, respectively) being better when compared to the first 50 implants (0.94 mm, 1.19 mm, and 3.48 degrees, respectively).29
While the present case report demonstrates the use of a fiduciary marker and thermoplastic stent, future applications of dynamic navigation surgery plan to involve the use of trace registration (TR) mapping technology. With TR, existing structures that are rigidly affixed to the patient's jaw (such as the natural dentition or existing implants) can serve as a natural fiduciary for the registration of the CBCT imaging to the patient by the navigation software. This eliminates the need for a thermoplastic stent or specialized CBCT scan with an artificial metal fiduciary marker captured in space during the CBCT imaging. The diagnostic CBCT imaging can be used for the planning and navigation surgery, thus simplifying the workflow process. However, because the micron tracker camera needs to be able to track the patient during the operation, a tag referred to as the "jaw tracker" must be connected to the jaw being operated on. Because the maxilla is a static/nonmovable bone in relation to the cranium, the patient may wear specialized glasses that lean against the nose-bridge and ears, carrying that tag. In the mandible, however, which is a movable/dynamic bone, a jaw tracker tag reference must be fixed to the dentition (or the jaw itself) as a method to track its position. TR technology in the mandible is accomplished with the use of a dual-cure composite resin bonding of the jaw tracker assembly (consisting of the jaw tag and bendable stainless-steel wire) to a natural tooth, crown, or abutment. The future use of TR technology is expected to simplify and streamline the dynamic navigation workflow process and facilitate adoption of navigation technology to the private practice armamentarium involving surgical implant placement.
Dynamic navigation for dental implants is in its infancy but provides some distinct advantages to freehand surgery or static guidance. As any technology application generally improves with time and innovation, dynamic navigation has a promising future. Further research with a variety of clinical applications is needed, and validated, controlled, blinded, and randomized studies are required to demonstrate efficacy of patient care.
Lastly, dynamic navigation technology provides a modality of medico-legal documentation and an unprecedented level of surgical accountability to ensure that prosthetically directed surgical outcomes are achieved. This is largely due to the navigation system's ability to record and save the surgical images and video. This approach can also ensure the concept of "collaborative accountability."5 As with any treatment modality that advances patient care, however, technology is not a substitute for sound judgment and experience, nor can it or should it undermine biologic principles of surgery and/or proper decision-making for predictable wound-healing dynamics.
Conclusion
Many factors contribute to implant success, with 3D implant positioning emerging as perhaps the most critical. Dynamic navigation is a promising advance in CBCT-guided surgery to help improve placement accuracy. The ability to attain real-time verification and validation of position accuracy holds great potential and may enhance surgical transparency and accountability to optimize patient outcomes.
Acknowledgment
The authors would like to acknowledge and thank Christopher K. Ching, DDS, of Glenview, Illinois, for his collaboration and prosthetic expertise on this case.
About the Authors
George A. Mandelaris, DDS, MS
Adjunct Clinical Assistant Professor, Department of Graduate Periodontics, University of Illinois, College of Dentistry, Chicago, Illinois; Private Practice, Chicago, Oakbrook Terrace, and Park Ridge, Illinois
Luigi V. Stefanelli, DDS, PhD
Professor, master of second level in implant surgery and master of second level of prosthesis, Sapienza University of Rome, Rome, Italy; Private Practice, prosthetics and dental implant surgery, Rome, Italy
Bradley S. DeGroot, DDS, MS
Private Practice, Chicago, Oakbrook Terrace, and Park Ridge, Illinois
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Brånemark PI, Adell R, Breine U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81-100.
2. Mecall RA, Rosenfeld AL. Influence of residual ridge resorption patterns on fixture placement and tooth position. 1. Int J Periodontics Restorative Dent. 1991;11(1):8-23.
3. Mecall RA, Rosenfeld AL. The influence of residual ridge resorption patterns on implant fixture placement and tooth position. 2. Presurgical determination of prosthesis type and design. Int J Periodontics Restorative Dent. 1992;12(1):32-51.
4. Mecall RA, Rosenfeld AL. Influence of residual ridge resorption patterns on fixture placement and tooth position, Part III: Presurgical assessment of ridge augmentation requirements. Int J Periodontics Restorative Dent. 1996;16(4):322-337.
5. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 1: diagnostics, imaging, and collaborative accountability. Int J Periodontics Restorative Dent. 2006;26(3):215-221.
6. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 2. rapid prototype medical modeling and stereolithographic drilling guides requiring bone exposure. Int J Periodontics Restorative Dent. 2006;26(4)347-353.
7. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 3. stereolithographic drilling guides that do not require bone exposure and the immediate delivery of teeth. Int J Periodontics Restorative Dent. 2006;26(5):493-499.
8. Mandelaris GA, Rosenfeld AL, King S, Nevins ML. Computer-guided implant dentistry for precise implant placement: combining specialized stereolithographically generated drilling guides and surgical implant instrumentation. Int J Periodontics Restorative Dent. 2010;30(3):275-281.
9. Mallaya SM, White SC. The nature of ionizing radiation and risks from maxillofacial cone beam computed tomography. In: Sarment D, ed. Cone Beam Computed Tomography: Oral and Maxillofacial Diagnosis and Applications. Hoboken, NJ: John Wiley & Sons, Inc; 2014:25-41.
10. Jacobson MW. Technology and principles of cone beam computed tomography. In: Sarment D, ed. Cone Beam Computed Tomography: Oral and Maxillofacial Diagnosis and Applications. Hoboken, NJ: John Wiley & Sons, Inc; 2014:1-24.
11. Tyndall DA, Brooks SL. Selection criteria for dental implant site imaging: a position paper of the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(5):630-637.
12. Rios HF, Borgnakke WS, Benavides E. The use of cone-beam computed tomography in management of patients requiring dental implants: an American Academy of Periodontology best evidence review. J Periodontol. 2017;88(10):946-959.
13. Mandelaris GA, Scheyer ET, Evans M, et al. American Academy of Periodontology best evidence consensus statement on selected oral applications for cone-beam computed tomography. J Periodontol. 2017;88(10):939-945.
14. Sarment DP, Al-Shammari K, Kazor CE. Stereolithographic surgical templates for placement of dental implants in complex cases. Int J Periodontics Restorative Dent. 2003;23(3):287-295.
15. Sarment DP, Sukovic P, Clinthorne N. Accuracy of implant placement with a stereolithographic surgical guide. Int J Oral Maxillofac Implants. 2003;18(4):571-577.
16. Tardieu P, Vrielinck L. Implantologie assistée par ordinateur: le programme SimPlantSurgiCase™ et le SAFE System™. Cas clinique: Mise en charge immédiate d'un bridge mandibulaire avec des implants transmuqueux. Implant. 2003;19:15-28.
17. Testori T, Robiony M, Parenti A, et al. Evaluation of accuracy and precision of a new guided surgery system: a multicenter clinical study. Int J Periodontics Restorative Dent. 2014;34(suppl 3):s59-s69.
18. Pikos MA, Magyar CW, Llop DR. Guided full-arch immediate-function treatment modality for the edentulous and terminal dentition patient. Compend Contin Educ Dent. 2015;36(2):116-128.
19. Luebbers HT, Messmer P, Obwegeser JA, et al. Comparison of different registration methods for surgical navigation in cranio-maxillofacial surgery. J Craniomaxillofac Surg. 2008;36(2):109-116.
20. Jayaratne YS, Zwahlen RA, Lo J, et al. Computer-aided maxillofacial surgery: an update. Surg Innov. 2010;17(3):217-225.
21. Block MS. Static and dynamic navigation for dental implant placement. J Oral Maxillofac Surg. 2016;74(2):231-233.
22. Somogyi-Ganss E, Holmes HI, Jokstad A. Accuracy of a novel prototype dynamic computer-assisted surgery system. Clin Oral Implants Res. 2015;26(8):882-890.
23. Jung RE, Schneider D, Ganeles J, et al. Computer technology applications in surgical implant dentistry: a systematic review. Int J Oral Maxillofac Implants. 2009;24 suppl:92-109.
24. Schneider D, Marquardt P, Zwahlen M, Jung RE. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin Oral Implants Res. 2009;20 suppl 4:73-86.
25. Monje A, Galindo-Moreno P, Tözüm TF, et al. Into the paradigm of local factors as contributors for peri-implant disease: short communication. Int J Oral Maxillofac Implants. 2016;31(2):288-292.
26. Cosyn J, Hooghe N, De Bruyn H. A systematic review on the frequency of advanced recession following single immediate implant treatment. J Clin Periodontol. 2012;39(6):582-589.
27. Block MS, Emery RW, Cullum DR, Sheikh A. Implant placement is more accurate using dynamic navigation. J Oral Maxillofac Surg. 2017;75(7):1377-1386.
28. Block MS, Emery RW, Lank K, Ryan J. Implant placement accuracy using dynamic navigation. Int J Oral Maxillofac Implants. 2017;32(1):92-99.
29. Stefanelli VL, DeGroot BS, Lipton DI, Mandelaris GA. Accuracy of a dynamic dental implant navigation system in a private practice. Int J Oral Maxillofac Implants. In press.