You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The most common developmental cyst of the neck is the thyroglossal duct cyst (TGDC). The TGDC accounts for 70% of congenital neck cysts and is thought to originate from the epithelial remnants of the embryologic thyroglossal tract.1 The presumed embryologic origin explains why these cysts can be found anywhere between the foramen cecum of the dorsal tongue and the suprasternal notch (Figure 1). Cysts above the hyoid are commonly found in the submental area. However, in 60% to 80% of cases, the TGDC occurs below the hyoid. TGDCs can be diagnosed in patients at any age, but 50% of cases occur in the first two decades of life. There is no sex predilection, although some studies indicate a slightly higher female predilection.2-4 Interestingly, about 15% of TGDCs are not clinically evident until after age 50 years.5
Embryologic Origins
The thyroid gland is the first of the endocrine organs to develop in the embryo. The thyroid anlage develops at the foramen cecum as a small mass of endoderm late in the fourth week of gestation; by the end of the fifth week, the thyroid has divided into two lateral lobes connected by an isthmus. The thyroglossal duct begins to atrophy during this fifth week. By the seventh week, the thyroid gland has reached its final position, just caudal to the cricoid cartilage.
Between weeks 7 and 10, the thyroglossal duct has, in most cases, completely atrophied. During its descent, the thyroid passes ventral to the hyoid bone, except in 30% of cases in which the duct is located dorsal to the hyoid.6 Fragments of the thyroglossal duct can be found in about 50% of individuals.5 These tissue remnants can develop into cysts, sinus tracts, or fistulae in later life. The hyoid bone is intimately related to the thyroglossal duct tract by dividing the tract into suprahyoid and infrahyoid portions. An intervening portion of the tract appears to pass posteriorly, anteriorly, or through the substance of the hyoid bone.7
Clinical Presentation
Typically, a TGDC presents on the midline of the ventral or anterior surface of the neck and is intimately associated with the strap muscles. It may move cranially upon tongue protrusion and swallowing. Approximately 10% to 24% of cysts are located lateral to the midline and usually to the left due to the anatomical location of the levator glandulae thyroideae muscle being normally located to the left of the midline.1,5,8,9 When classifying a TGDC, the vertical location may vary on the ventral surface of the neck. For example, there are intralingual and suprahyoid locations, with the latter including submental, thyrohyoid, and suprasternal cysts.2 Interestingly, if patients have an upper respiratory tract infection, they may report a sudden increase in the size of the cyst.10 Intralingual TGDCs may cause choking sensations, dysphagia, and dysphonia. There are cases in which a cutaneous or oral fistula may form as a result of suppuration, trauma, or inadequate surgical removal. When a lingual thyroid is present, it may be due to hypertrophy of ectopic thyroid tissue.1
Diagnosis
Proper imaging is important in confirming the diagnosis and determining the presence and location of the thyroid, and for a preliminary analysis of cyst composition. For the initial investigation, high-resolution sonography is the ideal approach. Lingual TGDCs require laryngoscopy or 3D computed-tomography imaging. Fine-needle aspiration can also be used to confirm the diagnosis of a TGDC and to detect a possible malignancy. Along with classifying the vertical location of the cyst, the shape and echo patterns can be utilized. The shape of the cyst can be either unilocular or multilocular. The echo pattern can be anechoic, hypoechoic, pseudosolid, or heterogenous. Anechoic cases are uncomplicated with no internal echoes. Homogeneously hyperechoic or pseudosolid echoes can represent the presence of cholesterol crystals, proteinaceous fluid, or keratin. Hypoechoic cysts have a lower amplitude than surrounding tissues and are predominantly cystic.5,11
TGDCs with a history of previous infections or hemorrhage appear as heterogeneous complex cysts with internal echoes.12 Possible differential diagnoses should include: dermoid cysts, sebaceous gland cysts, branchial cleft cysts, thyroid nodules, lingual thyroid, tuberculosis, lipomata, hypertrophic thyroid pyramidal lobe, benign or malignant lymph node pathology, salivary gland tumors, suprasternal thymic duct cysts, choristomata and foreign bodies, and carcinoma.1,2
Histology
Contents of the cystic lumen are described as mucoid, gelatinous, or purulent yellowish white to dark brown. In some cases cholesterol can also be detected in the luminal contents. Thyroid tissue can be found in the wall of the cyst, and it appears that the occurrence ranges from 25% to 65% of cases.13 This rather large degree of variability may be a result of the different number of sections or degrees of overall sampling of each specimen. Different types of epithelial linings may be found, ranging from squamous to pseudostratified ciliated columnar epithelium. Variations in the type of epithelium may result from metaplasia of ciliated epithelium into squamous epithelium as a result of infection or inflammation. It is also possible for squamous epithelium to undergo metaplastic differentiation into ciliated epithelium following aspiration or drainage of cyst fluid.1,7 Cysts that are located close to the foramen cecum tend to be lined with stratified squamous epithelium, and those that are more proximal to the thyroid gland tend to be lined with thyroidal acinar tissue.5 The lining of the tract may be smooth with slit-like or branched side extensions that spread into the surrounding connective tissue for varying distances.6,14 If sinuses are present, they tend to be sites of recurring inflammation and may not reveal an epithelial lining. Subjacent to the epithelial layer, varying degrees of an inflammatory infiltrate composed of neutrophils, plasma cells, and lymphocytes may be present; deeper tissues tend to be dense and fibrotic. Histologic sections of the central portion of the hyoid bone may show the epithelial-lined tract passing through the bone or the periosteum.3 In some cases, internal septae can be detected and the wall thickness is classified as either >2 mm or ≤2 mm.5
Cholesterol granulomas (CGs) can be found in the lumen of 13% of TGDCs.15 These CGs are nonspecific histologic entities consisting of fibrous granulation tissue with an abundant amount of cholesterol crystal deposits that form needle-shaped clefts. Foreign-body giant cells are also found surrounding the deposits of crystals. CGs have been found to be associated with chronic inflammation involving the middle ear.15 It is thought that CGs form secondary to hemorrhaging into the cyst lumen.15
Malignant Transformation
Malignancies occur in about 1% of all TGDC cases, indicating that it is an extremely rare event.16 Typically, no diagnosis is made prior to histological analysis; however, exceptions include some cases in which sudden rapid clinical growth takes place. Of the thyroglossal duct cases that are malignant, 85.5% are papillary thyroid adenocarcinoma, 6.6% are squamous cell carcinoma, 4.4% are mixed papillary and follicular thyroid adenocarcinoma, 2.2% are adenocarcinoma, and 1.1% are follicular thyroid adeno-carcinoma.16 Capsule invasion is a common occurrence, but metastasis to proximal lymph nodes is rare, occurring at a rate of 7.9%.16
The clinical location of thyroglossal ducts is along the midline of the neck, thus any carcinoma that may develop could metastasize to lymph nodes on either side of the neck.1 The most common area of distant metastasis is the lungs.17 Carcinomas arising in a TGDC may consist of a radiographically dense or enhanced mural nodule with or without calcification within the cystic cavity.5
Etiology
Various events could cause the fragmented remnants of a thyroglossal duct to initiate the formation of a TGDC. Inflammation is considered the most common etiology. Lymphoid tissue adjacent to remnants of the thyroglossal duct can repeatedly become inflamed with infections such as colds, mumps, measles, and peritonsillitis. Infants and children are most likely to be affected by infectious diseases, which could be why such a high rate of occurrence exists in this population. Another possibility is the retention phenomenon in which a blocked thyroglossal duct expands as a result of an accumulation of secretions.1
Treatment
The most successful treatment of a TGDC is the Sistrunk procedure.18-20 It requires the complete removal of all of the epithelium-lined tract that runs from the foramen cecum to the cyst. The duct above the hyoid is normally small and fragile. Upon surgical excision, this part of the duct can easily become separated, making it difficult to completely remove. To remove all of the duct, an approximately 4-mm margin of tissue would need to be created on all sides of the duct.18,19 This involves coring out the tissues between the hyoid bone and foramen cecum. The tract may pass above, through, or below the center portion of the hyoid bone, requiring that the central one-fourth of the hyoid be removed. The tissue above the hyoid is usually cored out until the foramen cecum is reached. The proper 45° angulation of dissection toward the foramen cecum should be followed.20 The classic Sistrunk procedure, first described in 1920, is associated with a 3% to 4% recurrence rate.21 The Sistrunk procedure involves removal of the TGDC and central portion of the hyoid bone. Sistrunk also emphasized the importance of total excision (ie, “coring out”) of all ductal tissues that lie between the foramen cecum and hyoid.21
Case Report
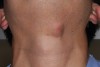
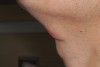
A 28-year-old man presented to the University of Missouri-Kansas City School of Dentistry with a movable ovoid mass of 2.0 cm x 1.2 cm located on the ventral surface of the neck at the level of the hyoid bone (Figure 2 and Figure 3). The patient first noticed what he called a “bump” at around age 19. Since then, the lesion had slowly grown. Periodic tenderness and swelling was noted coincidentally with an upper respiratory infection.
The patient’s medical and dental histories were noncontributory. The patient was referred to a plastic surgeon who rendered an initial diagnosis of a localized chronic infection related to an ingrown hair. In April 2014, the mass was excised to the depth of the tracheal cartilage. Histologic examination of the surgical specimen revealed a keratin granuloma with mixed inflammation and granulation tissue, lined by ciliated pseudostratified columnar and cuboidal epithelium. The results were inconclusive, but it was suggested that the histologic results could be that of a bronchogenic or brachial cleft cyst.
Six months after the surgery, the mass recurred, growing to its presurgical size. The patient was subsequently referred to an otolaryngologist (ear, nose, and throat) who rendered a diagnosis of a TGDC. A sonogram was performed to verify the presence of the thyroid.
In March 2015, a Sistrunk-like procedure was performed by the otolaryngologist. The standard core-out of tissue between the hyoid bone and the foramen cecum was not implemented. Instead, tissue was cored out coronal to the hyoid bone where the tract appeared to end. The two halves of the hyoid bone were not joined together because current evidence suggests that patients have a lower risk for recurrence.22
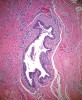
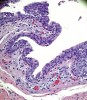
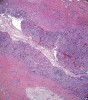
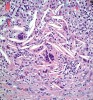
Histologic evaluation of this second surgical specimen showed no evidence of neoplasia or thyroid tissue. The cyst did not grossly involve the included bone specimen. The cystic area was lined by low cuboidal epithelium (Figure 4). At higher magnification, localized areas of the lumen were lined by a short, ciliated, columnar epithelium (Figure 5). Areas of cystic duct tissue were lined by a thin layer of squamous epithelium surrounded by granulation tissue and a dense inflammatory cell infiltrate (Figure 6). Inflamed connective tissue peripheral to the duct frequently exhibited cholesterol slits and multinuclear giant cells (Figure 7). Overall, these observations were consistent with a diagnosis of an inflamed TGDC. As of January 2016, no evidence of recurrence could be observed.
Conclusion
TGDCs are the most common developmental cysts of the neck that can be identified during a routine extraoral examination. While the occurrence rate of malignancy is low, the patient needs to be informed of that risk. Dental healthcare professionals have a responsibility to identify pathologies and refer patients to healthcare providers who may be able to provide further treatment as deemed necessary. This case report demonstrates a recurrence of a TGDC after a missed diagnosis, followed by proper surgical removal, and no evidence of recurrence at 10-months post-surgery.
ABOUT THE AUTHORS
Nicholas J. Fujii, DDS
Private Practice, Periodontics and Implantology, Honolulu, Hawaii
Tanya M. Gibson, DDS
Assistant Professor, Department of Oral and Maxillofacial Pathology, University of Missouri-Kansas City School of Dentistry, Kansas City, Missouri
Keerthana M. Satheesh, DDS, MS
Chair, Department of Periodontics, University of Missouri-Kansas City School of Dentistry, Kansas City, Missouri
Charles M. Cobb, DDS, MS, PhD
Professor Emeritus, Department of Periodontics, University of Missouri-Kansas City School of Dentistry, Kansas City, Missouri
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Allard RH. The thyroglossal cyst. Head Neck Surg. 1982;5(2):134-146.
2. Shah R, Gow K, Sobol SE. Outcome of thyroglossal duct cyst excision is independent of presenting age or symptomatology. Int J Pediatr Otorhinolaryngol. 2007;71(11):1731-1735.
3. Neville BW. Developmental defects of the oral and maxillofacial region. In: Neville BW, ed. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders; 2009:35-36.
4. O’Connell M, Grixti M, Harmer C. Thyroglossal duct carcinoma: presentation and management, including eight case reports. Clin Oncol (R Coll Radiol). 1998;10(3):186-190.
5. Chou J, Walters A, Hage R, et al. Thyroglossal duct cysts: anatomy, embryology and treatment. Surg Radiol Anat. 2013;35(10):875-881.
6. Chandra RK, Maddalozzo J, Kovarik P. Histological characterization of the thyroglossal duct tract: implications for surgical management. Laryngoscope. 2001;111(6):1002-1005.
7. Stahl WM Jr, Lyall D. Cervical cysts and fistulae of thyroglossal tract origin. Ann Surg. 1954;139(1):123-128.
8. Androulakis M, Johnson JT, Wagner RL. Thyroglossal duct and second branchial cleft anomalies in adults. Ear Nose Throat J. 1990;69(5):318-322.
9. Nachlas NE. Thyroglossal dust cysts. Ann Otol Rhinol Laryngol. 1950;59(2):381-390.
10. Noyek AM, Friedberg J. Thyroglossal duct and ectopic thyroid disorders. Otolaryngol Clin North Am. 1981;14(1):187-201.
11. Ahuja AT, King AD, King W, Metreweli C. Thyroglossal duct cysts: sonographic appearances in adults. Am J Neuroradiol. 1999;20(4):579-582.
12. Ahuja AT, Wong KT, King AD, Yuen EH. Imaging for thyroglossal duct cyst: the bare essentials. Clin Radiol. 2005;60(2):141-148.
13. Adelchi C, Mara P, Melissa L, et al. Ectopic thyroid tissue in the head and neck: a case series. BMC Res Notes. 2014;7:790-794.
14. Horisawa M, Niinomi N, Ito T. Anatomical reconstruction of the thyroglossal duct. J Pediatr Surg. 1991;26(7):766-769.
15. Shvili I, Hadar T, Sadov R, et al. Cholesterol granuloma in thyroglossal cysts: a clinicopathological study. Eur Arch Otorhinolaryngol. 2009;266(11):1775-1779.
16. Gordini L, Podda F, Medas F, et al. Tall cell carcinoma arising in a thyroglossal duct cyst: a case report. Ann Med Surg (London). 2015;4(2a):129-132.
17. Pellegriti G, Lumera G, Malandrino P, et al. Thyroid cancer in thyroglossal duct cysts requires a specific approach due to its unpredictable extension. J Clin Endocrinol Metab. 2013;98(2):458-465.
18. Wagner G, Medina JE. Excision of thyroglossal duct cyst: the Sistrunk procedure. Oper Tech Otolaryngol. 2004;15(3):220-223.
19. Gallagher TQ, Hartnick CJ. Thyroglossal duct cyst excision. Adv Otorhiolaryngol. 2012;73(1):66-69.
20. Sistrunk WE. The surgical treatment of cysts of the thyroglossal tract. Ann Surg. 1920;71(2):121-122.
21. Lin ST, Tseng FY, Hsu CH, et al. Thyroglossal duct cyst: a comparison between children and adults. Am J Otolaryngol. 2008;29(2):83-87.
22. Ubayasiri KM, Brocklehurst J, Judd O, Beasley N. A decade of experience of thyroglossal cyst excision. Ann R Coll Surg Engl. 2013;95(4):263-265.