You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Initial Examination and Evaluation
A patient’s candidacy for oral appliance therapy is determined based on careful evaluation of the dentition, periodontium, oral soft tissues, masticatory muscles, and TMJ. A sufficient number of teeth (ideally, eight in each arch) without decay or faulty restorations and of sufficient height to retain the oral appliance must be present, although mandibular advancement devices (MADs) can be supported by dental implants (Figure 1).1 Any active periodontal disease should be treated. The alveolar bone level should be adequate to withstand forces applied by the appliance. The presence of temporomandibular disorders per se is not a contraindication to oral appliance therapy. However, some patients will not be able to tolerate jaw advancement, while others may benefit from it.2 Based on the sleep study report and the orofacial examination, the dentist determines whether the patient is a good, guarded, or poor candidate for MAD therapy.
Impressions and Records
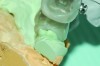
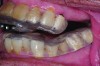
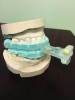
Accurate impressions of the upper and lower dentition are required for custom fabrication of a MAD. Because some appliances extend beyond the teeth, it is recommended that the impression capture at least 3-4 mm of soft tissue surrounding the teeth. The lower impression must capture the distal surfaces of the most distal teeth on both sides of the arch. The MAD must be fabricated to extend to include these surfaces; otherwise, diastemas may form with the adjacent mandibular teeth (Figure 2 through Figure 4).
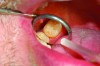
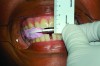
An interocclusal record known as a construction bite is taken to establish the initial jaw advancement of the MAD.3 The construction bite is commonly taken as a percentage (eg, 60-80%) of the patient’s maximum protrusion (Figure 5), a fixed number of millimeters (eg, 3) less than the patient’s maximum protrusion, or at an advanced yet comfortable position of the mandible. Other strategies for taking the construction bite also have been advocated.
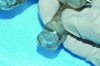
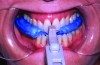
Regardless of the manner in which the bite is taken, it is imperative that the dental laboratory fabricate the MAD according to the bite. Failure to use the construction bite in the fabrication of a MAD can result in a nonfunctional appliance (Figure 6). If the minimum jaw advancement of the appliance is smaller than that of the construction bite, it may not be possible to achieve the level of jaw advancement needed to treat the patient. This is because many MADs have a limited range over which jaw advancement can be adjusted. However, some newer models incorporate features that effectively extend the limited range of jaw advancement permitted, circumventing this problem. Usually in cases in which the initial jaw advancement of the appliance differs from that of the construction bite, the MAD will need to be returned to the dental laboratory for correction (Figure 7).
The thickness of the construction bite determines the separation between the upper and lower teeth (the vertical dimension) when the MAD is worn. The dentist must assure that a minimum separation (of approximately 3-4 mm) is allowed between all upper and lower teeth to accommodate the thickness of the upper and lower components of most appliances. A thicker record is sometimes taken to create more space for a large tongue. Records also will be thick posteriorly for patients with deep overbites or if an anterior opening between the upper and lower components is indicated to enable the passage of air for mouth breathing. The literature suggests that the efficacy of the MAD is not altered by changes of approximately 10 mm in the vertical dimension.4 However, patients tend to prefer appliances that separate the teeth no more than necessary to accommodate the two layers of acrylic. If the vertical dimension is too great, lip competency will be lost, which is poorly tolerated by some patients and often contributes to mouth breathing and complaints of dry mouth. The dental laboratory should contact the dentist if the vertical dimension of the construction bite must be altered in order to fabricate the MAD (Figure 8 through Figure 10).
Dentists may indicate teeth, or tooth surfaces, on the work order form that should be engaged minimally by the appliance. These teeth may have fragile restorations (eg, anterior teeth with facial ceramic veneers or incisal composite restorations) or minimal bony support. These areas should be coated with block-out material prior to fabrication of the appliance.
Selection of Appliance
The literature does not support any one adjustable MAD design as being more efficacious in normalizing sleep respiration than another.5,6 However, the individual patient’s anatomy characteristics may dictate which appliances are likely to be better or worse choices (Table 1). A primary factor in appliance selection is identifying one that will be comfortable and used nightly. Unfortunately, this can be difficult to anticipate in advance of fabrication.
Delivery of Appliance
During delivery, the dentist assures that the MAD seats fully on the teeth, is sufficiently retentive, and advances the jaw according to the construction bite. In general, the appliance should not alter the patient’s dental midlines from the relationship observed when the teeth or casts are intercuspated. An exception is when the patient’s jaw naturally deviates to one side during protrusion. In this case, different alignments will be observed by the dental technologist when the casts are articulated with the construction bite and when hand articulated without the bite. If any question exists about the accuracy of the construction bite, the dental technologist should contact the dentist for confirmation.
If the cameo surfaces of the upper and lower components come into contact when the patient closes, it is desirable that the contact be even across the arch. Thus, if the patient bruxes during sleep, both TMJs will be loaded similarly.
Patients typically are instructed to wear the MAD all night long, inserting it before bedtime, after brushing and flossing. Depending on the material used, the appliance is inserted after soaking in warm water (thermoflex materials) or directly without warming (non-thermoflex materials). The MAD is removed upon wakening and cleaned according to the instructions provided by the manufacturer of the appliance. Non-abrasive cleaners usually are recommended, including those effervescent cleaners that are safe for orthodontic appliances and removable partial dentures. The MAD is stored dry or in clear water, depending on the manufacturer’s instructions. As the MAD is durable medical equipment, instructions on its use and cleaning should be noted in the patient’s chart.
Upon removal of the MAD, the mandible often remains slightly advanced and the patient’s teeth do not maximally intercuspate upon biting. Many dentists instruct patients on morning exercises that help reposition the jaw back to the maximum intercuspation position. It is thought that the exercises may slow development of the dental side effects of therapy (see Part III of this series at insidedentaltech.com/idt908). Some exercises involve the use of a thermoplastic template of the patient’s pre-treatment occlusion, or a bite tab or block, to help guide the mandible backward into position (Figure 11).
Pre-treatment photographs of the teeth may be taken at the appliance delivery appointment. Some dentists also advocate obtaining a lateral skull film (cephalographic image) or a cone-beam computed tomography (CBCT) scan. These images provide a baseline by which the positions of the teeth can be compared after wearing the appliance.
Calibration of Appliance and Verification of Efficacy
By Patient Report
Patients typically are instructed to wear their MAD for 1-4 weeks before any additional adjustments are made. Thereafter, adjustments are made by the dentist at follow-up appointments (most commonly) or by the patient at home. The jaw is advanced in small increments (typically 0.3-1 mm) to further decrease the patient’s reports of symptoms of SDB, including snoring, poor or unrefreshing sleep, and residual excessive daytime sleepiness.7,8 Different clinicians advance the jaw at different rates to achieve symptomatic improvement, but usually not more than 1 mm per week. Also, patients’ tolerance for aggressive advancement varies.
Upon good symptomatic improvement, patients with a diagnosis of primary snoring are placed on recall. However, patients with a diagnosis of OSA require further testing. Even with positive symptomatic improvement, many of these patients will continue to have OSA.9
By Home Sleep Apnea Testing
After symptomatic improvement is achieved, some dentists administer home sleep apnea testing (HSAT).9,10 Unlike use of HSAT for the diagnosis of SDB (see Part I of this series at insidedentaltech.com/849), this method is used to determine whether the frequency of respiratory events (apneas, hypopneas, and/or oxygen desaturations), referred to as the respiratory event index (REI), is reduced below a desired target level.11,12 Ideally, the mandible is advanced after each home sleep apnea test until all respiratory events are eliminated without causing discomfort. It may be helpful to obtain a baseline HSAT study (without the appliance) to compare to the data from the treatment position HSAT(s). If the test is limited to pulse oximetry, the REI is not calculated and auto-scored measures of blood oxygen saturation will often be normal in patients who still have residual mild OSA. The use of HSAT by the dentist does not eliminate the need for follow-up evaluation by the patient’s physician to evaluate the efficacy of therapy.
By In-Laboratory Sleep Study (PSG)
Other dentists do not employ home sleep apnea testing but send the patient back to the referring physician after symptomatic improvement is achieved.13,14,15 The physician may order a follow-up laboratory PSG or HSAT to assess the efficacy of the therapy in normalizing the AHI and oxygen saturation. The dentist should encourage adjustment of the MAD to improve its efficacy during these tests and provide instructions on how to advance the jaw if respiratory events are detected during the first 30-90 minutes of sleep. If the MAD is not adjusted as indicated, the patient may remain inadequately treated. Often, the dentist meets directly with the sleep technologist to discuss adjustment of the appliance prior to the study. Some dentists prefer that the technologist make the adjustments. Other dentists recommend that the patient adjust the appliance upon being awakened. More than one adjustment may be needed during the night, particularly if the patient’s OSA is position dependent or worse in REM sleep. At the end of the study and for medico-legal reasons, the MAD is typically adjusted back to the level at which the patient presented to the laboratory upon arrival. The sleep study data are scored separately for each adjustment made to the appliance. The American Board of Dental Sleep Medicine considers a patient to be a successful responder if the patient experiences symptomatic improvement; the AHI is reduced to fewer than 10 events/hour; and the AHI is reduced ≥50% of the pretreatment value.
Pre-treatment Calibration
Recently, the possibility of systematically manipulating jaw position during a sleep study prior to fabrication of a MAD has drawn interest. The aim is to determine whether the individual patient likely will respond to a mandibular advancement appliance, and if so, to determine the level of advancement predicted to effectively treat the patient. This protocol can be implemented in the sleep laboratory by the technologist using either a temporary prefabricated adjustable oral appliance, or a remotely controlled mandibular positioner.16,17 For use of the latter, trays of impression material are fit to the patient’s upper and lower teeth. The tray handles are engaged by a small, motorized device that the sleep technologist controls from the data observation room. The lower tray is advanced in small steps until respiratory events are adequately eliminated. The adjusted temporary appliance, or the set of trays locked into the calibration position, serve as the construction bite for the dental laboratory’s subsequent fabrication of a custom MAD ordered by the patient’s sleep dentist (Figure 12). This promising new protocol has not been widely endorsed by third-party payers to date, which limits its demand. Moreover, it remains unclear whether the patient requires a follow-up sleep study when the oral appliance is fabricated in this manner.
Recall
After the final adjustment of the MAD and verification of efficacy, the patient is placed on recall. Yearly appointments are recommended to evaluate the integrity of the appliance and its fit to the teeth, dental side effects, oral health, patient compliance to therapy, and symptoms that suggest worsening of the patient’s SDB. Consensus opinion is that custom-fabricated MADs should last at least 3 years.6 As appliances age, retention often decreases, particularly for MADs made of dual laminate materials, and hairline fractures develop in the acrylic (Figure 13).
Financial Considerations
Dental sleep medicine manages a medical, not dental, condition. As such, medical diagnostic and procedure codes are used in documentation and insurance claims. Medical, not dental, insurance reimbursement is sought. An oral appliance is not viewed as a dental device, such as a bite guard or stabilization splint, but rather as durable medical equipment (DME). Accordingly, some third-party payers, such as the Centers for Medicare and Medicaid Services (Medicare), view dentists as DME suppliers; they require that the physician provide an order for durable medical equipment (DME) prior to delivery of the device, and restrict reimbursement to only a subset of available appliance types on the market, those that are Pricing, Data Analysis, and Coding (PDAC) administration approved.
The criteria required of MADs for PDAC approval are not based on current scientific evidence and have led to exclusion of some excellent, clinically validated appliances for use by patients who are Medicare recipients.6 In some cases, functionally unnecessary components have been added to MADs to meet PDAC criteria, resulting in two similar FDA-cleared versions of the same appliance (Figure 14). Dentists may not be aware of these distinctions.
The dental laboratory can help assure that an approved appliance is ordered for the patient. For example, work order forms should be designed to clearly indicate which appliance designs are PDAC approved. Moreover, dental laboratory technicians should call the dentist if a PDAC unapproved appliance is ordered for a patient who is Medicare-eligible based on age information provided on the order form.
Concluding Remarks
Dental sleep medicine is one of the fastest growing disciplines in dentistry as dentists continue to play an important role in identifying and treating patients with snoring and OSA. This field is expanding as new technologies are introduced each year. Research assessing the efficacy of oral appliances also increases each year and provides further support for use of these devices in treating a serious health problem (OSA). Oral appliance therapy can provide a life-changing experience for patients. It can improve their sleep, health, mood, and relationships.
Dental laboratory technologists are a vital part of the team that provides effective care for patients suffering from OSA. Clear communication between the dentist and the dental laboratory is vital for fabricating a high-quality, comfortable, and effective appliance for each patient.
About the Authors
Gregory K. Essick,DDS, PhD
Professor
Department of Prosthodontics and Center for Pain Research and Innovation
School of Dentistry
University of North Carolina
Chapel Hill, NC
Andrew R. Blank, AAS, BS
Dental Student
East Carolina University School of Dental Medicine
Greenville, NC
Jamison R. Spencer, DMD, MS
Director
The Center for Sleep Apnea and TMJ
Boise, ID
Jonathan A. Parker, DDS
Owner
Clinical Director
Snoring and Sleep Apnea Dental Treatment Center
Edina, MN
References
1. Hoekema A, de Vries F, Heydenrijk K, Stegenga B. Implant-retained oral appliances: a novel treatment for edentulous patients with obstructive sleep apnea-hypopnea syndrome. Clin Oral Implants Res. Jun 2007;18(3):383-387.
2. Perez CV, de Leeuw R, Okeson JP, et al. The incidence and prevalence of temporomandibular disorders and posterior open bite in patients receiving mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath. Mar 2013;17(1):323-332.
3. George PT. Selecting sleep-disordered-breathing appliances. Biomechanical considerations. J Am Dent Assoc. Mar 2001;132(3):339-347.
4. Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. Sep 15 2002;166(6):860-864.
5. Balevi B. There is no one mandibular advancement device design that fits all for the management of obstructive sleep apnea. J Am Dent Assoc. Mar 2014;145(3):280-282.
6. Scherr SC, Dort LC, Almeida FR, et al. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: A report of the American Academy of Dental Sleep Medicine. Journal of Dental Sleep Medicine. 2014;1(1):39-50.
7. Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. May 2001;163(6):1457-1461.
8. Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. Apr 1997;52(4):362-368.
9. Fleury B, Rakotonanahary D, Petelle B, et al. Mandibular advancement titration for obstructive sleep apnea: optimization of the procedure by combining clinical and oximetric parameters. Chest. May 2004;125(5):1761-1767.
10. Levendowski DJ, Morgan TD, Patrickus JE, et al. In-home evaluation of efficacy and titration of a mandibular advancement device for obstructive sleep apnea. Sleep Breath. Sep 2007;11(3):139-147.
11. Moore KE, Prinsell JR, Parker JA, et al. Academy of Dental Sleep Medicine--position statement. Dental sleep medicine and portable monitoring, August 2005. Sleep Breath. Dec 2005;9(4):189-192.
12. Love AL, Kuna ST. Home sleep testing and sleep apnea: a review for dentists. Journal of Dental Sleep Medicine. 2015;2(2):45-52.
13. Dieltjens M, Vanderveken OM, Heyning PH, Braem MJ. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev. Apr 2012;16(2):177-185.
14. Holley AB, Lettieri CJ, Shah AA. Efficacy of an adjustable oral appliance and comparison with continuous positive airway pressure for the treatment of obstructive sleep apnea syndrome. Chest. Dec 2011;140(6):1511-1516.
15. Almeida FR, Parker JA, Hodges JS, Lowe AA, Ferguson KA. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J Clin Sleep Med. Jun 15 2009;5(3):198-204.
16. Remmers J, Charkhandeh S, Grosse J, et al. Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. Sleep. Oct 2013;36(10):1517-1525, 1525A.
17. Roy S. In pilot study, prefab device predicts positive responses to custom oral appliances. Sleep Review Magazine. 2014(June).
Fig 1. Edentulous patient treated with a MAD (SUAD Herbst appliance: http://www.strongdental.com/). The upper component is fit and contoured similar to the patient’s full upper denture. The lower component is retained by two dental implants with Locator® abutments (http://www.zestanchors.com/ ).
Fig 2. Inadequate posterior extension of a MAD. The posterior extent of the MAD failed to capture the distal surfaces of the mandibular second molars. All third molars were absent.
Fig 3. Eight months later, diastemas had developed between the mandibular second and first molars.
Fig 4. The appliance was returned to the dental lab for correction of the distal extensions, using the original dental casts. The diastemas closed in response to the anteriorally directed force applied to the mandibular second molars (not shown).
Fig 5. Clinical recording of a construction bite. Registration material captures the relationship between the upper and lower teeth when the mandible is held forward using a George Gauge ( http://www.greatlakesortho.com/ ). The MAD is fabricated to hold the jaw in this position when first inserted, before any adjustment.
Fig 6. MAD fabricated without casts being mounted according to the construction bite included with the case. Forward advancement of the mandible was not possible due to mechanical binding and blockage of the upper and lower tray components.
Fig 7. MAD fabricated without adherence to the jaw position registered by the construction bite. The minimum advancement of the MAD approximated the patient’s maximum protrusion and 3-4 mm greater than that prescribed. Patient could not tolerate appliance.
Fig 8. Illustration of required changes in the vertical dimension prescribed by the construction bite. Note that the anterior vertical dimension of the construction bite was 6.5 mm
Fig 9. The MAD was fabricated with an anterior vertical dimension 1.5 mm greater than the construction bite (Figure 16).
Fig 10. Increased vertical dimension may be required to accommodate the thickness of the upper and lower tray components of a MAD. This is particularly true when the Curve of Spee is pronounced. Prior to fabrication of the appliance, the dental lab should contact the dentist when a change in the vertical dimention is anticipated.
Fig11. Devices used in the morning to help reposition the mandible backward until the teeth maximally intercuspate. Top row, from left to right: A.M. Aligner (http://tapintosleep.com/ ), anterior bite positioner made of Thermacryl (http://tapintosleep.com/ ). Bottom row, from left to right: Good Morning Positioner (https://www.smlglobal.com), Morning Repositioner (http://www.strongdental.com/).
Fig 12. Casts positioned in titration trays (MATRx: http://zephyrsleep.com/) prior to fabrication of a MAD. The position of the lower tray with respect to the upper, was systematically advanced during a sleep study until sleep respiration improved a targeted amount. The calibrated titration trays are used in the same manner as a construction bite (Figure 13) in the fabrication of a MAD.
Fig 13. The aged MAD. Note breakdown, fractures and pitting of the acrylic.
Fig 14. Altered MAD design to comply with PDAC criteria, requiring that an appliance have a ‘hinge’. Shown are two version of the TAP®3 appliance (http://tapintosleep.com/) : standard TAP®3 (left) and TAP®3 elite double bar (right). Only the appliance on the right is approved for Medicare recipients.