You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Throughout patients’ lives—from toddler stage, to teenage years, to adulthood— oral care providers are there at regular intervals to assess and treat them as their particular condition requires. As time passes, the ongoing progression of disease in dental hard and soft tissue is a multifactorial downward spiral. Oral care providers must fully understand the disease process, then proactively intervene to slow or even stop its progress before the situation requires more extensive treatment. This is the concept of “proactive intervention dentistry.”
This article focuses on dental hard tissue and the various elements and techniques that have been found beneficial in reversing and controlling the caries process. These tools can be used proactively when the patient requires supplementary help in maintaining hard-tissue health.
Dental Caries and Demineralization
Dental caries is a multifactorial disease that is caused by the interaction of dietary sugars, dental biofilm, and the host’s dental tissue within the oral environment.1 It is the net result of consecutive cycles of demineralization and remineralization at the interface between the biofilm and the tooth surface. Demineralization is caused by the acid production of oral bacteria after sugar consumption. In response to this cariogenic challenge, hydroxyapatite crystals are dissolved from the subsurface. Remineralization is the natural repair process for noncavitated lesions and relies on calcium and phosphate ions, assisted by fluoride, to rebuild a new surface on the existing crystal remnants in the subsurface. The remineralized crystals are less acid-soluble than the original mineral.2
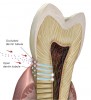
Caries progression is slow because saliva is supersaturated with calcium and phosphate ions under normal physiological conditions (pH 7). However, as bacteria continue to produce acid during sugar challenges, the plaque pH falls to between 4.5 and 5.5, and the driving force is shifted to mineral dissolution.1 The lower the pH, the higher the concentrations of calcium and phosphate required to reach saturation with respect to hydroxyapatite. In other words, as the pH is lowered, the saturation point of the minerals in the surrounding fluid is changed. This is called the “critical pH,” and it is the point at which equilibrium exists. There is no mineral dissolution nor any mineral precipitation. The critical pH of hydroxyapatite is approximately 5.5; that of fluorapatite is approximately 4.5. This varies with individual patients. Below critical pH, demineralization occurs, while above critical pH, remineralization occurs (Figure 1 and Figure 2). The critical pH is significantly higher for children than adults, meaning that at a given pH, children are more prone to demineralization. This puts children at greater risk for demineralization than adults.3
The Use of Fluoride
It has been known since the 1980s that fluoride controls caries mainly through its topical, not systemic, effect.1 Fluoride’s mechanism of control is fourfold:
1. Fluoride inhibits demineralization. If fluoride is present in plaque fluid when bacteria produce acids, it will penetrate along with the acids at the subsurface, adsorb to the apatite crystal surface, and protect the crystals from dissolution.4 The coating makes the characteristics of the crystal similar to those of fluorapatite, with a critical pH of 4.5, so that no demineralization takes place until the pH reaches this level. Fluoride present in solution at low levels among the enamel crystals can markedly inhibit dissolution of the tooth mineral by acid.5 This fluoride comes from topical sources, such as drinking water, and products such as toothpastes and varnishes. The fluoride incorporated developmentally and systemically into the normal tooth mineral is insufficient to have a measurable effect on acid solubility.5
2. Fluoride enhances remineralization. When the pH returns to above 5.5, the saliva, which is supersaturated with calcium and phosphate, provides a driving force for mineral to go back into the tooth.5 Fluoride will adsorb to the surface of the partially demineralized crystals and attract calcium ions. The newly formed surface veneer will preferentially take up fluoride from the solution surrounding the crystals and exclude carbonate.5 Fluoride speeds up the growth of the new surface by bringing calcium and phosphate ions together and is preferentially included in the end-product, which now has a lower solubility. The remineralized surface is more resistant to acid challenges.
3. Some fluoride sources inhibit essential bacterial activity. Fluoride cannot cross the bacterial cell wall in its ionized form (F). However when acids are produced, F combines with hydrogen (H) to form hydrogen fluoride (HF), which rapidly diffuses into the bacterial cell.6 Once inside the cell, the HF dissociates again and releases fluoride ions, which interfere with essential enzyme activity in the bacterium.
4. Intraoral reservoirs. Fluoride can be retained in intraoral reservoirs after application of a fluoride vehicle such as toothpaste, varnish, or restorative materials and be released into the saliva over time.7 Fluoride can be deposited on dental hard tissue, become bound to the oral mucosa, or be retained within the dental plaque. The clinical significance of fluoride retention, especially in dental plaque, is that it can be released during cariogenic challenges when it is most needed to decrease demineralization and enhance remineralization.1
Other Remineralization Therapies
The action of fluoride in remineralization is the standard against which other newer therapies are compared. The ideal remineralization material8:
• diffuses or delivers calcium and phosphate into the subsurface.• does not deliver an excess of calcium.
• does not favor calculus formation.
• works at an acidic pH.
• works in xerostomic patients.
• boosts the remineralizing properties of saliva.
• shows a benefit over fluoride.
Three major remineralization technologies available in the dental marketplace are Recaldent™ (CPP-ACP), NovaMin®, and tricalcium phosphate (TCP). These technologies, as well as the use of xylitol, are discussed below.
Recaldent (CPP-ACP)
This technology combines phosphoproteins from milk with amorphous calcium phosphate (ACP). ACP has been shown to produce a thin surface coating of hydroxyapatite when applied topically. This is purely a surface phenomenon. It is fundamentally different from the remineralization of enamel subsurface lesions, which requires actual penetration of ions into the enamel.8
The addition of casein phosphopeptide (CPP) makes Recaldent (Recaldent Pty Ltd., www.recaldent.com) more effective than ACP alone. Within milk, the CPP stabilizes calcium and phosphate ions through the formation of complexes that are available for intestinal absorption. The same concept has been applied to Recaldent, which has bioavailable calcium and phosphate in the appropriate form for remineralization of subsurface lesions in enamel, not just the surface. CPP also localizes the ACP in the dental plaque biofilm.9 By stabilizing calcium phosphate in solution, CPP creates a high calcium and phosphate ion concentration gradient that drives the ions into the subsurface lesions and achieves high rates of remineralization.10 Recaldent has been incorporated into solutions, gums, lozenges, and creams.
NovaMin
NovaMin (GlaxoSmithKline, www.gsk.com) is described as an inorganic amorphous calcium sodium phosphosilicate (CSPS). It belongs to a class of materials known as “bioactive glasses.” NovaMin and other CSPS materials were originally developed as bone regenerative materials in the early 1970s. Until the invention of bioactive glass, all biomaterials were made to be as inert as possible in the human body.11 The discovery that a synthetic biomaterial could form a chemical bond with bone proved that biomaterials could be engineered to interact with the body, and it was not necessary or even advantageous to minimize these interactions. Bioactive glasses facilitate hydroxyapatite deposition when exposed to fluids containing calcium and phosphate.12 In the presence of water or saliva, NovaMin rapidly releases sodium ions. This increases the local pH and initiates the release of calcium and phosphate. Numerous studies have shown that the NovaMin particles act as reservoirs to continuously release calcium and phosphate ions into the local environment. This may continue over many days.13 As the reactions and deposition of calcium-phosphate complexes continue, this layer crystallizes to hydroxycarbonate apatite, which is chemically and structurally similar to biological apatite.14 NovaMin has been incorporated into toothpastes, gels, and prophy pastes.
A novel system for NovaMin delivery is through an air-polishing unit (Figure 3). This method has been developed as an improved cleaning modality with the added benefit of desensitization and smoothing of surface irregularities. It has been shown to significantly reduce dentin permeability and completely occlude exposed dentinal tubules.15 NovaMin powder also has positive remineralization effects on partially and completely demineralized models of dentin. The treatment decreases surface roughness, promoting a smoother and less plaque- and stain-retentive surface (Figure 4).16
Tricalcium Phosphate (TCP)
TCP is a bioactive formulation of tricalcium phosphate and simple organic ingredients. It works synergistically with fluoride to produce superior remineralization of enamel subsurface lesions, as compared with using fluoride alone.17 In toothpaste formulation, a protective barrier is created around the calcium, allowing it to coexist with the fluoride ions. During toothbrushing, the toothpaste comes into contact with saliva, dissolving the barrier and releasing calcium, phosphate, and fluoride. When TCP is incorporated into a 5% sodium fluoride (NaF) varnish, results for microhardness and acid resistance improve.18
Xylitol
Xylitol is one of a number of non-sugar sweeteners permitted for use in foods throughout the world. It is a sugar alcohol that has been shown to have noncariogenic as well as cariostatic effects.19 More recently, it has been shown that the habitual use of xylitol is associated with a significant reduction in caries incidence and increased tooth remineralization.20 Cariogenic bacteria process xylitol very poorly, producing little acid or plaque. This decreases caries incidence as well as promotes colonization of less virulent strains of bacteria that can ferment xylitol.
Dental caries is an infectious, transmissible, bacterial disease. Most children acquire the bacteria (predominantly Streptococcus mutans) from their mothers/caregivers by salivary contact during the emergence of the primary teeth between the ages of 6 to 30 months.21 This is the “discrete window of infectivity.” After the initial colonization of S. mutans, the successful establishment of other bacteria on the tooth surfaces is impaired. It has been demonstrated that a reduction of S. mutans in the saliva of mothers has resulted in the delayed acquisition of S. mutans in their children.22 Remarkably, studies have shown that the habitual chewing of xylitol gum by mothers can decrease the caries incidence in their children by preventing the transmission of S. mutans (Figure 5).23
Research has found that chewing xylitol gum decreases caries incidence significantly up to at least 5 years after the xylitol therapy has been discontinued.24 Children who chew xylitol gum have demonstrated a significantly lower caries progression and a significant number of caries lesion reversals, suggesting that remineralization has occurred.25 The efficacy of xylitol candies has been shown to be equivalent to that of xylitol gum.26 The dental literature suggests that a minimum of 5 g to 6 g and three exposures per day from chewing gum or candies is required for clinical effect.27
Bioactive Restorative Materials
When the enamel and dentin no longer have sufficient structure to maintain their mineral framework, cavitation takes place and remineralization is insufficient. The tooth now requires preparation and restoration. Although many of the restorative materials are inert with respect to the biological tissues of the tooth, some are bioactive (interact with or affect the biological tissues). These materials can effectively work with the dental hard tissues to harden and “heal” them. Three bioactive restorative materials are discussed below.
Glass-Ionomer Cements
Glass-ionomer cements (eg, Riva Self Cure, SDI, www.sdi.com; GC Fuji IX™, GC America, Inc., www.gcamerica.com; ChemFil® Rock, DENTSPLY Caulk, www.dentsply.com) are especially valuable for initial carious lesions, abfractions/erosions/abrasions, and for caries control in patients who are at high risk for caries.28 Glass ionomers have a true chemical bond with dental tissue. They are bioactive, encourage remineralization of the surrounding tooth structure, and prevent bacterial microleakage through the ion-exchange adhesion that they develop with both enamel and dentin.29 This creates a new, ion-enriched material at the tooth–glass-ionomer interface. This material consists of phosphate and calcium ions from the dental tissues, and calcium (or strontium), phosphate, and aluminum from the glass-ionomer cement.29 This remineralization creates a harder dentin surface (Figure 6).30 Restoration failure is usually cohesive, leaving the ion exchange layer firmly attached to the cavity wall and the dentinal tubules sealed and protected against bacteria penetration.31 Fluoride is the catalyst for remineralization, aided by calcium (or strontium) and phosphate. The pattern of release for all the ions in glass-ionomer cement is similar, and this is enhanced by a low pH.32
The bioactive remineralizing effect of glass ionomers occurs on two different aspects of the tooth: 1) The outer surface of the restoration is exposed to oral fluids and plaque with which it has a continuous exchange of ions.33 Wear resistance is low at placement and steadily increases with time and ion uptake. 2) The inner surface of the restoration, next to the preparation, is isolated from the oral environment. The continuous flow of dentinal fluid creates a wet environment, which is conducive to the exchange of ions. At placement, there is a significant release of ions from the cement; they combine with similar ions from the dentinal fluid and promote remineralization. After the glass ionomer sets, there is a continuous low-level ion exchange, which accounts for the remineralization that is found clinically.31
Giomers
Giomers are the latest category of hybrid restorative materials; they are bioactive. Giomer technology represents the true hybridization of glass ionomers and composite resins, offering the fluoride release and recharge of glass ionomers and the esthetics, physical properties, and handling of composite resins.34
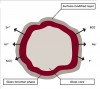
The giomer concept is based on pre-reacted glass (PRG) technology; pre-reacted glass particles—surrounded by a glass-ionomer phase—are enclosed within a polyacid matrix (Figure 7). Studies show dentin remineralization occurs at the preparation surface adjacent to the giomer.35 Giomers are also able to take up extra fluoride (after using fluoride toothpaste, rinse, varnish, etc.) in the oral fluids and then act as a reservoir until the fluoride is needed. This is termed “fluoride release and recharge” (Figure 8). Giomers release and recharge fluoride efficiently and significantly better than compomers36 and composite resins—although not as well as glass ionomers.37 Giomer fissure sealants have superior recharge and release of ions when compared to resin sealants. Hence, they can work actively to decrease demineralization and increase remineralization in young teeth at their most caries-susceptible stage.38
Giomers resist plaque formation.39 A “material film layer” forms on the surface of the giomer after contact with saliva. This layer consists of aluminum, silica, strontium, and other ions that originate from the PRG fillers and act to inhibit bacterial adhesion. Clinical performance of giomers has been tested against that of hybrid resin composites, and giomers have been found to compare favorably for all criteria.40
Tricalcium Silicate Cement
A new bioactive calcium-silicate-based product (Biodentine™, Septodont, www.septodontusa.com) that has been designed as a “dentin replacement” material can be used in endodontic repair (root perforations, apexification, resorptive lesions), pulp capping, and as a dentin replacement in restorative dentistry. It was formulated by taking mineral trioxide aggregate (MTA)-based endodontic repair cement technology, improving its physical and handling properties, and creating a dentin replacement material with significant reparative qualities.
Biodentine penetrates the dentinal tubules, forming tag-like structures that create a micromechanical lock with the tooth, and then begins to stimulate reparative dentin. The product has been shown to enhance the formation of reparatory dentin and create a dense dentin barrier after direct pulp capping,41 as well as heal damaged pulp fibroblasts.42 The material seems to have the potential to heal pulps, enabling the avoidance of what may have been inevitable endodontic treatment in the past.
Daily Oral Hygiene
The demineralization and remineralization of dental hard tissue is in a dynamic, ever-changing flux in the oral environment. While the techniques and materials described above aid in decreasing demineralization and increasing remineralization of these tissues, no preventive or restorative material can be totally effective in controlling further caries development without a vigorous at-home daily oral hygiene routine. Especially important is the removal of plaque (biofilm) from hard-to-access areas, where it can remain undisturbed and cause the greatest damage. This is predominantly in the subgingival and interproximal areas.
A toothbrush, either a power or manual type, is considered the first line of defense for cleaning teeth and is the primary vehicle for delivering topical fluoride. String floss is considered essential for removing the biofilm responsible for interproximal caries. However, a systematic review43 of string floss use in reducing interproximal caries has not shown significant results. Flossing was shown to be effective in reducing interproximal caries only when performed by dental professionals and only on children with little exposure to fluoride. Studies on adolescents performing self-flossing under supervision showed no improved results. The review found no studies on adult subjects and flossing efficacy in caries reduction. For an oral hygiene device to be useful for caries reduction, three things are needed: 1) the device must be effective in removing plaque; 2) it must be easy to use properly; and 3) it must be pleasant and not burdensome to use in order to promote compliance.
Over-the-counter fluoride rinses, xylitol products, and dentifrices with additives to enhance mineralization are available for daily use. However, not all products will provide a clinical benefit, thus dentists need to review the research on individual products. For instance, xylitol efficacy is based on concentration and frequency exposure, therefore occasionally chewing gum with xylitol does not constitute intervention.
A water flosser has been shown to be more effective than string floss for removing plaque by accessing the interdental and subgingival areas. An ex vivo study showed a 99.9% removal of biofilm from the tooth surface when treated with a water flosser for 3 seconds at medium pressure.44 Clinical studies on water flosser use in combination with manual toothbrushing have reported a 75% plaque removal after a single use. Most importantly, the results showed 92% removal of plaque in the approximal area.45 Unlike string floss, a water flosser is easy to use effectively at a young age, requiring far less dexterity. For orthodontic patients, an orthodontic tip attachment on a water flosser was found to be three times more effective than flossing with a floss threader for biofilm reduction.46 Specific tips can be effective for particular oral hygiene requirements such as cleaning around indirect restorations like veneers, implants, crowns, and bridges. For patients with xerostomia, a water flosser can be used to flush out accumulated food debris, which not only decreases caries risk but enhances comfort and quality of life.
Compliance and acceptance of the water flosser has been extensively studied. In one study, the subjects felt that using the water flosser was pleasant and their mouths felt cleaner.47 Other research found 74% of the subjects had continued using the water flosser a year after the study was completed because it “stimulated the gums and made the teeth feel cleaner.”48 At the end of a study with adolescent orthodontic patients, 92% of the participants said they would continue to use the water flosser daily or frequently, compared to 58.8% who said they would continue to use string floss.46 Its efficacy, ease of use, and high compliance make the water flosser an oral hygiene device that can be effective for caries reduction, flushing out plaque in hard-to-access areas—subgingivally and interproximally—where debris can stay undisturbed and cause the most damage over time.
Conclusion
The medical model of proactive intervention described is becoming the paradigm in dental care. This should be an integral part of daily practice and not relegated to only the “preventive” side of dentistry. The multifactorial disease process of demineralization and caries can be slowed or even stopped before more extensive treatment becomes necessary. Oral care providers have the tools and techniques available, including oral hygiene care, that have been found effective in reversing and controlling the caries process. They can be used proactively to maintain hard-tissue health throughout a patient’s life.
References
1. Buzalaf MAR. Fluoride and the oral environment. Monographs in Oral Science Series. Basel, Switzerland: Karger Publishers; 2011;22:97-114.
2. Featherstone JD. Dental caries: a dynamic disease process. Aust Dent J. 2008;53(3):286-291.
3. Anderson P, Hector MP, Rampersad MA. Critical pH in resting and simulated whole saliva in groups of children and adults. Int J Paediatr Dent. 2001;11(4):266-273.
4. Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol. 1999;27(1):31-40.
5. ten Cate JM, Feathersone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(3):283-296.
6. Van Louveren C. The antimicrobial action of fluoride and its role in caries inhibition. J Dent Res. 1990;69 Spec No:676-681.
7. Pessan JP, Alves KM, Ramires I, et al. Effects of regular and low-fluoride dentifrices on plaque fluoride. J Dent Res. 2010;89(10):1106-1110.
8. Walsh LJ. Evidence that demands a verdict: latest developments in remineralization therapies. Australasian Dental Practice. 2009;March/April:48-59.
9. Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health-chemistry and clinical applications. Curr Pharm Des. 2007;13(8):793-800.
10. Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilization calcium phosphate solutions. J Dent Res. 1997;76(9):1587-1595.
11. Hench LL. Biomaterials. Science. 1980;208(4446):826-831.
12. Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (Novamin): remineralization potential. Adv Dent Res. 2009;21(1):35-39.
13. Damen JJ, Ten Cate JM. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. J Dent Res. 1992;71(3):453-457.
14. Gandolfi MG, Silvia F, H PD, et al. Calcium silicate coating derived from Portland cement as a treatment for hypersensitive dentine. J Dent. 2008;36(8):565-578.
15. Sauro, S, Watson TF, Thompson I. Ultramorphology and dentine permeability changes induced by phophylactic procedures on exposed dentinal tubules in middle dentine. Med Oral Patol Oral Cir Bucal. 2011;16(7):1022-1030.
16. Wang Z, Jiang T, Sauro S, et al. Dentine remineralization induced by two bioactive glasses developed for air abrasion purposes. J Dent. 2011;39(11):746-756.
17. Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Enhancing remineralization of subsurface enamel lesions with functionalized fTCP. In: Bourg H, Lisle A, eds. Biomaterials Developments and Applications. Hauppauge, NY: Nova Science Publishers; 2010:353-374.
18. Flanigan PJ, Vang F, Pfarrer AM. Remineralization and acid resistance effects of 5% NaF varnishes. J Dent Res. 2010;89(spec iss B):383.
19. Maguire A, Rugg-Gunn AJ . Xylitol and caries prevention – is it a magic bullet? Br Dent J. 2003;194(8):429-436.
20. Mäkinen KK. Sugar alcohols, caries incidence and remineralization of caries lesions: a literature review. Int J Dent. 2010;2010:981072.
21. Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a descrete window of infectivity. J Dent Res. 1993;72(1):37-45.
22. Kohler B, Andreen I. Influence of caries-preventive measures in mothers on cariogenic bacteria and caries experience in their children. Arch Oral Biol. 1994;39(10):907-911.
23. Isokangas P, Soderling E, Pienihakkinen K, Alanen P. Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res. 2000;79(11):1885-1889.
24. Isogangas P, Makinen KK, Tiekso J, Alanen P. Long term effect of xylitol chewing gum in the prevention of dental caries: a follow up 5 years after termination of a prevention program. Caries Res. 1993;27(6):495-498.
25. Kandelman D, Gagnon G. Clinical results after 12 months from a study of the incidence and progression of dental caries in relation to consumption of chewing gum containing xylitol in school preventive programs. J Dent Res. 1987;66(8):1407-1411.
26. Alanen P, Isokangas P, Gutmann K. Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidermiol. 2000;28(3):218-224.
27. Milgrom P, Ly KA, Rothen M. Xylitol and its vehicles for public health needs. Adv Dent Res. 2009;21(1):44-47.
28. Mount GJ. An Atlas of Glass-Ionomer Cement: A Clinicians Guide. 2nd ed. London, England: Martin Dunitz Publishers; 1994.
29. Mount GJ. Adhesion of glass-ionomer cement in the clinical environment. Oper Dent. 1991;16(4):141-148.
30. McIntyre JM, Cheetham J, Dalidjan M. Ionic exchange between Riva Self Cure GIC and demineralized dentine. Paper presented at: International Association of Dental Research (IADR). June 30, 2006; Brisbane, Australia. Abstract 2078.
31. Mount G. Glass ionomers: advantages, disadvantages, and future implications. In: Davidson CL, Mjor IA, eds. Advances in Glass Ionomer Cements. Hanover Park, IL: Quintessence Publishing; 1999:269-293.
32. Ngo H, Marino V, Mount GJ. Calcium strontium, aluminum, sodium and fluoride release from four glass-ionomers [abstract]. J Dent Res. 1998:77(641). Abstract 75.
33. De Moor RJG, Verbeek RM, De Maeyer EA. Fluoride release profiles of restorative glass ionomer formulations. Dent Mater. 1996;12(2):88-95.
34. Koirālā S, Yap A. A Clinical Guide to Direct Cosmetic Restorations with Giomer. Leipzig, Germany: Dental Tribune International; 2008.
35. Miyauchi T, Akimoto N, Ohmori M, et al. The effect of Giomer restorative materials on demineralized dentin. Paper presented at: International Association of Dental Research (IADR). July 17, 2010; Barcelona, Spain. Abstract 4540.
36. Dhull KS, Nandlal B. Comparative evaluation of fluoride release from PRG-composites and compomer on application of topical fluoride: an in-vitro study. J Indian Soc Pedod Prev Dent. 2009:27(1):27-32.
37. Okuyama K, Murata Y, Pereira PN, et al. Fluoride release and uptake by various dental materials after fluoride application. Am J Dent. 2006:19(2):123-127.
38. Shimazu K, Ogata K, Karibe H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler . Dent Mater J. 2011;30(6):923-927.
39. Honda T, Yamamoto K, Hirose M, et al. Study on the film substance produced from S-PRG filler. Japanese Journal of Conservative Dentistry. 2002:45(Autumn):42.
40. Tian FC, Gao X-J. Clinical performance of Giomer restorative system. Poster presented at: International Association of Dental Research (IADR). July 14-17, 2010; Barcelona, Spain.
41. Shayegan A. RD 94 Etude #PC08-001, Etude de RD 94 comme agent pulpaire dans le cadre de pulpotomie et coiffage direct sur les dents lactéales de cochon. Report RD RA DEV 94-006. 2009.
42. About I. Effects des matériaux bioactifs Biodentine™ et Calcipulpe® sure les étapes précoces de la régénération dentaire. Report RD RA DEV 94-013. 2009.
43. Hujoel PP, Cunha-Cruz J, Banting DW, Loesche WJ. Dental flossing and interproximal caries: a systematic review. J Dent Res. 2006:85(4):298-305.
44. Gorur A, Lyle DM, Schaudinn C, Costerton JW. Biofilm removal with a dental water jet. Compend Contin Educ Dent. 2009:30(special issue 1):1-6.
45. Sharma NC, Lyle DM, Qaquish JG, Schuller R, Comparison of two interdental cleaning devices on plaque removal. J Clin Dent. 2012;23:17-21.
46. Sharma NC, Lyle DM, Qaqish JG, et al. The effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent orthodontic patients with fixed appliances. Am J Ortho Dentofacial Orthop. 2008;133(4):565-571.
47. Hoover DR, Robinson HB. The comparative effectiveness of a pulsating oral irrigator as an adjunct in maintaining oral health. J Periodontol. 1971;42(1):37-39.
48. Lainson PA, Bergquist JJ, Fraleigh CM. A longitudinal study of pulsating water pressure cleaning devices. J Periodontol. 1972;43(7);444-446.
About the Authors
Fay Goldstep, DDS
Private Practice
Markham, Ontario