You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Dentists have been placing posterior composites for almost 30 years. Early posterior composites were unsuccessful, as bonding was limited and excessive wear occurred.1 Original posterior composites consisted of 10µ glass fillers, which plucked out as resin wore around and excessive force was applied to them. Inadequate bonding combined with differences in composite-to-tooth thermal coefficient of expansion resulted in recurrent decay.
Modern composites are successful when appropriate materials, techniques, and restorative situations are selected.2 Posterior composites must resist forces applied in the oral environment, including mastication, parafunctional habits, clenching, and bruxism, or fracture will occur. They must resist hot, cold, and chemical application, which can result in breakdown of material or microleakage at cavosurface areas. Proper bonding techniques provide strength, minimize microleakage, and reduce postoperative sensitivity.3
In order to properly select composite materials a dentist must know composite science and history as well as restorative techniques. Proper selection results in highly esthetic and long-lasting restorations.
History and Science
Silicate was the original white filling.4 It was used for non-load-bearing restorations in which esthetics was important. Silicate restorations were highly successful, as they lasted a long time. However, these restorations stained rapidly.
Acrylics were used in dental prostheses dating back to the early 1900s but could not be used as restorations because they created pulpitis and periodontitis.5,6 Rafael Bowen modified acrylics with epoxy to create BIS-GMA resin, which when modified with other materials such as cross-linking polymers and glass particles became dental composite.7,8 Modern composite is highly successful on posterior teeth when placed in small restorations where moderate force is applied.
Composite science is complex and includes resin chemistry, oxygen inhibition, filler, and methods of cure. Understanding science prepares dentists to create long-lasting, esthetic restorations and enables composite selection and restorative techniques to be done properly.
Resin Chemistry
Understanding composite science is critical for material selection. BIS-GMA resin is a mixture of bisphenol A and glycidyl metyhacrylate diluted with glycol dimethacrylate. Cross-linking of polymer chains is accomplished with triethylene glycol dimethacrylate and ethylene glycol dimethacrylate. Monomers, or single molecules, are joined together to form polymers, or long chain molecules, by production of free radicals. Studies on acrylic shrinkage reveal shrinkage of more than 24%.9 Single molecules are held together with gravity of van der Waals forces at 4 angstroms. Molecules join into long chains at distances of 1.9 angstroms. Distances vary depending on molecule size. The change in distance results in dramatic shrinkage in amounts unacceptable to restore teeth. Resin is modified with fillers and chemicals that cross-link polymer chains to reduce shrinkage to acceptable levels.
Oxygen Inhibition
Oxygen inhibits resin cure, and composite surfaces that touch air will not cure. Several microns of material on composite surfaces do not cure, which allows subsequently placed layers to join together. Layering techniques are important to minimize negative effects of shrinkage.10 The oxygen-inhibited layer of bonding agents allows composite to bond. On the last layer placed, the outer layer of composite does not cure, so a surface must be removed or covered and then cured. Covering composite with glycerine and final curing is a popular technique.
Oxygen inhibition weakens composite when it is entrapped during mixing, which can occur during composite production as fillers and resins are mixed. Mixing of self-cure and dual-cure components entraps air. Mixing tints to change composite color also weakens composite.
Fillers
Addition of fillers such as glass spheres reduces the amount of resin present, resulting in less shrinkage. Filler shape, size, surface, load, and optical index affect composite handling characteristics, esthetics, strength, wear, and ability to polish.11
Filler-to-resin interface is critical to composite strength and wear. Composite will fracture if excessive force is applied. A fracture starts in resin and continues until it strikes particles. It works around particle surfaces and continues. Larger particles, aggregated particles, and particles that have surface modification with chemicals such as silane or surface roughness make fracture propagation more difficult and composite stronger. Stresses that contribute to breakdown of filler-to-resin interfaces occur from resin shrinkage, differences in thermal coefficient of expansion, and modulus of elasticity.12
Initiation, Propagation, and Termination
Conversion of monomer to polymer is initiated by energy from free radicals. Free radicals are produced from a chemical reaction such as a catalyst and base, heat, or light-activated chemicals. Light-sensitive chemicals such as diketones and camphorquinones are activated by blue light, which results in a slow cure. Amines are added to accelerate cure time.13 Propagation or continued reaction occurs up to termination, ultimately resulting in about 80% of the resin being converted. A more complete conversion is accomplished by removing oxygen, which inhibits cure, and applying heat and/or pressure. Interestingly, 100% cure results in a composite that is too brittle, therefore cure conversion is in the low 90 percentile.
Light activation initiates chemical cure with an intermediate gel phase. When the gel phase occurs later in the reaction, less shrinkage takes place. The gel phase can be moved to later in the application through use of various light applications such as ramped or pulsed.
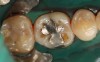
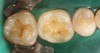
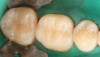
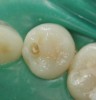
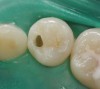
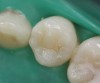
Case 1 (Figure 1 through Figure 6) reveals a mandibular first molar with severe wear and a defective amalgam. The amalgam and decay were removed, the tooth was prepared, and bonding to enamel and dentin was completed. An initial layer of composite was placed and cured, followed by many subsequent layers to minimize the negative effects of shrinkage. The composite was shaped and initial polishing was completed. Occlusion was adjusted, final shaping was accomplished and polishing was completed.
Classification
Composite is classified by filler, viscosity, and method of cure. Filler particles in most composites are glass that is ground or precipitated. The resulting particles are varied in size based on a bell curve. When filler particles are greater than 1µm, the composite is referred to as a macrofill. When filler particles are less than 1µm, the composite is referred to as a microfill. A mixture of particle sizes is referred to as a hybrid. Filler particles are visible if they are greater that than 1µm. As resin wears and particles become exposed, they can be seen by the eye, though this is not as critical in posterior teeth.
The newest development in particles is the nanoparticle, which is about 5 nm to 450 nm. Nanoparticles have been produced for many decades, but they stuck together or agglomerated in such a fashion that they were not useful for improving dental composites. Recently, companies have advanced techniques that allow particle separation, and now nanoparticles are used as the primary filler in some composites. Other composites use them to fill between larger glass particles. The resulting composites produce shrinkage of 2% or less.
Composite viscosity is defined as flowable, regular, or packable. Flowable allows easy wetting or adaptation to surfaces and placement into small areas. It consists primarily of resin with little filler, consequently there is excessive shrinkage and mechanical properties are diminished. Packable composite, which was introduced as a material that would feel similar to amalgam and to create contacts similarly, is high in filler content with little resin. It is difficult for layers of packable composite to join together, as there is little resin to be oxygen-inhibited. It is also difficult to wet surfaces with packable composite, and, because air is trapped, a liner of glass ionomer or flowable is usually placed.14
Composite is also classified by method of cure. Usually, self-cure composite mixes components as a catalyst and base to initiate a chemical cure. Mixing components incorporates air, which results in a weaker composite. This occurs because entrapped oxygen inhibits material cure where contacted. Dual-cure composite mixes components to initiate chemical cure and has light-sensitive chemicals that further initiate cure. Dual-cure composites are used as cementing mediums for posts or crowns in areas that might not be reached with light activation. Light-cure composite uses blue light to activate chemicals such as diketones or camphorquinones to produce free radicals and initiate cure. Lastly, heat cures composite and resins, and when combined with other techniques such as vacuum and pressure, produces a more thorough cure.15
Composite Selection
Composite selection is based on material strength, wear, and shrinkage; esthetic characteristics such as translucency, fluorescence, chroma, value, and filler; handling characteristics, ability to hold its shape and not slump during placement, depth of cure, and ambient light sensitivity; capacity to color modify and characterize; ease of adjustment, sculpture, and polish; and cost.16
Strength of materials is defined by compressive, shear, tensile, modulus of elasticity, yield, and torsional strength. Compressive strength of composite is adequate for restoration. Most restoration mechanical failure comes from fracture. While in vitro studies give some indication of material strength, they do not replicate the effects of constant repetitive forces as seen in vivo, which leads to fatigue. Analyzing forces and controlling them is critical to success. Composite should not be used in high-stress areas. Sharp opposing cusps should be recontoured. Patients who exhibit signs of bruxing should wear a nightguard. Diet and parafunctional forces should be evaluated.17,18
Filler particle size and other characteristics are important for strength. Fracture starts on a resin surface propagating until it strikes a particle. It works around a particle and continues on to the next particle. Larger particles have more surface area and impede fracture propagation. Surfaces that are roughened or treated with chemicals such as silanes also inhibit fracture propagation. Original posterior composites utilized 10µm-sized glass spheres. Wear was so excessive that they all failed rapidly.1
Larger particles or particles that aggregate make composite stronger. Unfortunately, larger particles produce other problems. Particles are exposed as resin wears on composite surfaces. As larger particles are exposed and force is applied, they pluck out. Wear occurs as a result of abrasive, adhesive, and chemical forces onto filler, in addition to fatigue.12
Shrinkage is an important consideration when selecting a composite material. Composite shrinkage may cause cusp fracture or enamel fracture, or produce latent stress resulting in microleakage and cavosurface staining.19 The negative effects of shrinkage are controlled by material selection; technique; placing small increments, which results in small amounts of shrinkage; proper bonding techniques; and proper isolation.
Composite selection is critical to esthetic results. Analysis of hue, chroma, value, translucence, and florescence is not easy. It is ideal to have composite materials that create a chameleon effect, with the composite picking up color around it and showing through color from under it. Filler material and size also affect results. The eye can see fillers greater than 1µ and as resin wears surfaces begin to look rough with larger particles. Fillers also need to have an optical index of 1.5 to provide translucency.20,21
The ability to work with a material is critical. Posterior composite that sticks to instruments more than tooth structure, slump on placement, harden quickly from ambient light, or do not wet tooth surfaces easily are too difficult to work with and usually discarded. A sublayer of glass ionomer or flowable composite placed on tooth surfaces aids with wetting.
Composite is adjusted and finished after curing. Outer layers of cured composite are uncured, since, as mentioned earlier, oxygen inhibits cure. Composite is overbuilt and the outer layers removed during final shaping and occlusal adjustment. Alternatively, composite is covered with materials like glycerin and cured. Composite added to a restoration after the outer layer is removed creates a weak interface. Final polishing and smoothing should be completed with routine rubber wheels, points, brushes, and pastes.22
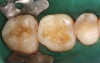
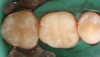
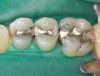
Case 2 (Figure 7 through Figure 11) depicts a series of defective amalgams on the first molar and biscupids. The amalgams were removed, the decay was excavated and cracks were eliminated. Bonding was completed on dentin and enamel. The teeth were matrixed and composite was built with layering techniques. To control contours, each tooth was completed individually, and then the next one was done. Composite was shaped, occlusal adjustment was completed, and final polishing was done.
Evaluation of Restorative Situations
Beyond improved appearance posterior composite provides several other advantages as well as disadvantages for tooth restoration. Composite is bonded to tooth structure, resulting in adequate retention and resistance to forces in the oral environment. Bonded composite supports moderately weak tooth structure. Pits and fissures are sealed with composite so extension for prevention is not required, as occurs with amalgam. Drilling gains access to caries, caries is removed, and unsupported enamel is finished, which results in much less tooth loss than when other materials are used. More remaining tooth structure results in stronger teeth and less trauma to pulp, thereby reducing the need for endodontics and reducing tooth fracture.23
Composite, however, is not as strong as amalgam, gold, or porcelain and does not last as long.24 It tends to crack easier than other materials, thus evaluating forces applied to a tooth is critical. A composite restoration should not extend beyond one-third the intercuspal width. Larger restorations are successful if less force is applied; however, it is difficult to analyze force intensity and the effect of fatigue. Molars create as much as five times the force of bicuspids. A person with a low-angle jaw creates much more force than a person with a high-angle jaw. Larger muscles create more force than smaller muscles. Ten times normal forces are created during grinding. Dietary habits determine the amount of force required to chew. History of existing restorations indicates forces applied to teeth. Shape and type of contact from opposing cusps are also factors, such that a sharp-pointed cusp creates many more pounds per square inch than a cusp with a three-point contact.25,26
Technique
Posterior composites require tooth preparation; bonding to enamel and dentin; composite placement and curing; composite shaping, including occlusal adjustment; and composite polishing. Tooth preparation requires cleaning and roughening tooth surfaces, access to caries, caries removal, and finishing of enamel margins so unsupported enamel or thin areas of composite do not fracture. Pits and fissures require opening if caries is suspected.
Bonding is accomplished on enamel and dentin. Enamel requires exposure to acid to dissolve inorganic structure quicker than organic structure, resulting in the classic honeycomb effect and micromechanical retention. Acid is applied separately as phosphoric acid or as part of an acidic one-step bonding agent. Dentin is bonded with materials that remove the smear layer or penetrate through it. Bonding enamel and dentin minimizes the extent to which microleakage occurs and reduces postoperative sensitivity. Microleakage results from composite curing shrinkage, differences in thermal coefficient of expansion, and differences in modulus of elasticity during chewing or grinding.
Composite is placed in small increments, touching as few walls as possible. Various restorative designs create different amounts of stress. As the author explains to patients, if a room is filled with composite and the composite is cured, the ceiling pulls against the floor, the right wall against the left wall, and the back wall against the front wall such that even 2% shrinkage would collapse the room. If the composite is just placed on the floor, it pulls down toward the floor with little stress. If composite is placed on the floor and just touches a little of an adjacent wall, there is little stress created. Restorative preparations are similar and can have from one to five walls as seen on surface defects or Class 1 preparations.
The amount of stress created from composite shrinkage during curing has been studied extensively. The “C” factor describes the amount of stress. It is the number of walls composite touches when cured over the number of walls it is not touching. Interestingly, a five-wall defect filled in bulk with composite and cured results in a gap under the filling and extreme pain for potentially many months.27,28
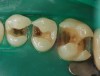
Case 3 (Figure 12 through Figure 15) on x-ray reveals a cavity in the mesial pit of the mandibular first bicuspid. Access was gained, caries removed, and bonding completed. A difficult five-wall defect required that small increments of composite be placed.
Conclusion
Composite selection requires understanding of material science and the requirements of various restorative situations. Materials must maximize esthetics and be easily placed, cured, shaped, and finished. They should be placed in situations where restoration is small and forces are average.
References
1. Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21(1):9-20.
2. Turkun LS, Aktener BO. Twenty-four-month clinical evaluation of different posterior composite resin materials. J Am Dent Assoc. 2001;132(2):196-203.
3. Mackenzie L, Shortall AC, Burke FJ. Direct posterior composites: a practical guide. Dent Update. 2009;36(2):71-80.
4. Christensen GJ. The need for caries-preventive restorative materials: J Am Dent Assoc. 2000;131(9):1347-1349.
5. Altintas SH, Yondem I, Tak O, Usumez A. Temperature rise during polymerization of three different materials. Clin Oral Investig. 2008;12(3):283-286.
6. Stanley HR, Swerdlow H, Buonocore MG. Pulp reactions to anterior restorative materials. J Am Dent Assoc. 1967;75(1):132-141.
7. Bowen RL. Use of epoxy resins in restorative materials. J Dent Res. 1956;35(3):360-369.
8. Bowen RL. Dental filling material comprising vinyl silane treated fused silica and a binder consisting of the reaction product of bis phenol and glycidyl aerylate. US patent 3,066,112. November 27, 1962.
9. Cowpwethwaite, GF, Foy JJ, Malloy, MA. The nature or the cross-linking matrix found in dental composite filling materials and sealants. In Gebelein GC, Koblitz FF, eds. Biomedical and dental applications of polymers. Polymer science and technology. Vol 14. New York, NY: Plenum Press; 1981:379-385.
10. Ghivari S, Chandak M, Manvar N. Role of oxygen inhibited layer on shear bond strength of composites. J Conserv Dent. 2010;13(1):39-41.
11. Cross M, Douglas WH, Fields RP: The relationship between filler loading and particle size distribution in composite resin technology. J Dent Res. 1983;62(7):850-852.
12. Lutz F, Phillips RW, Roulet JF, Sectos JC. In vivo and in vitro wear of potential posterior composites. J Dent Res. 1984;63(6):914-920.
13. Truffier-Boutry D, Demoustier-Champagne S, Devaux J, et al. A physic-chemical explanation of the post-polymerization shrinkage in dental resins. Dent Mater. 2006;22(5):405-412.
14. Shi L, Wang X, Zhao Q, et al. Evaluation of packable and conventional hybrid resin composites in Class I restorations: three-year results of a randomized, double-blind and controlled clinical trial. Oper Dent. 2010;35(1):11-19.
15. Willems G, Lambrechts P, Braem M, et al. A classification of dental composites according to their morphological and mechanical characteristics. Dent Mater. 1992;8(5):310-319.
16. Ferracane JL. Resin composite—state of the art. Dent Mater. 2011;27(1):29-38.
17. Piwowarczyk A, Ottl P, Lauer HC, Büchler A. Laboratory strength of glass ionomer cement, compomers, and resin composites. J Prosthodont. 2002;11(2):86-91.
18. Lu H, Lee YK, Oguri M, Powers JM. Properties of a dental resin composite with a spherical inorganic filler. Oper Dent. 2006;31(6):734-740.
19. Shawkat ES, Shortall AC, Addison O, Palin WM.: Oxygen inhibition and incremental layer bond strengths of resin composites. Dent Mater. 2009 Nov;25(11):1338-46.
20. Lee YK, Lim BS, Rhee SH, et al. Changes of optical properties of dental nano-filled resin composites after curing and thermocycling. J Biomed Mater Res B Appl Biomater. 2004;71(1):16-21.
21. Bowen RL. Compatibility of various materials with oral tissues. I. The components in composite restorations. J Dent Res. 1979;58(5):1493-1503
22. Chalifoux PR. Comprehensive composite restoration. Inside Dentistry. 2006;2(4):56-58.
23. Chalifoux PR. Aesthetic guidelines for posterior composite restorations. Pract Periodontics Aesthet Dent. 1996;8(1):39-48.
24. Goldstein GR. The longevity of direct and indirect posterior restorations is uncertain and may be affected by a number of dentist-, patient-, and material-related factors. J Evid Based Dent Pract. 2010;10(1):30-31.
25. Lee MR, Cho BH, Son HH, et al. Influence of cavity dimension and restoration methods on the cusp deflection of premolars in composite restoration. Dent Mater. 2007;23(3):288-295.
26. Abe M, Medina-Martinez RU, Itoh K, Kohno S. Temporomandibular joint loading generated during bilateral static bites at molars and premolars. Med Biol Eng Comput. 2006;44(11):1017-1030.
27. van Dijken JW. Durability of resin composite restorations in high C-factor cavities: a 12-year follow-up. J Dent. 2010;38(6):469-474.
28. Nikolaenko SA, Lohbauer U, Roggendorf M, et al. Influence of c-factor and layering technique on microtensile bond strength to dentin. Dent Mater. 2004;20(6):579-585.