You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
While the prevalence of dental caries in the US has shown a dramatic decline in recent decades that correlates with the advent of fluoridated water, numerous tooth cleaning devices and products, antibiotic treatment, and professional intervention, dental caries remains the number one chronic childhood disease. A high incidence continues to be reported in adults, particularly in seniors.1,2 In their recent meta analysis, Griffen et al found older adults experienced the same, if not more, caries than children.3 Furthermore, perhaps as little as 10% of late adolescents and young adults have no caries;4-11 almost all adults (> 95%) are reported to have enamel and root-surface caries. Complete edentulism caused by caries occurs in 25% of people. This data seems to be at odds with the fact that dental caries—and most oral diseases—is largely preventable if patients practice good oral hygiene and receive professional plaque biofilm removal. However, those preventive methods demand optimal patient cooperation and motivation, which is difficult both to obtain and maintain.12 Unfortunately for many people, caries cavitation can be a lifelong illness13 marked by a never-ending cycle of restoration and almost inevitable re-treatment, particularly in, but certainly not limited to, underserved and undereducated populations.
Learning Objectives
After reading this article, the reader should be able to:
• discuss dental caries as a disease.
• explain caries management by risk assessment.
• discuss the concept of caries infiltration.
• describe the caries infiltration treatment process.
While the prevalence of dental caries in the US has shown a dramatic decline in recent decades that correlates with the advent of fluoridated water, numerous tooth cleaning devices and products, antibiotic treatment, and professional intervention, dental caries remains the number one chronic childhood disease. A high incidence continues to be reported in adults, particularly in seniors.1,2 In their recent meta analysis, Griffen et al found older adults experienced the same, if not more, caries than children.3 Furthermore, perhaps as little as 10% of late adolescents and young adults have no caries;4-11 almost all adults (> 95%) are reported to have enamel and root-surface caries. Complete edentulism caused by caries occurs in 25% of people. This data seems to be at odds with the fact that dental caries—and most oral diseases—is largely preventable if patients practice good oral hygiene and receive professional plaque biofilm removal. However, those preventive methods demand optimal patient cooperation and motivation, which is difficult both to obtain and maintain.12 Unfortunately for many people, caries cavitation can be a lifelong illness13 marked by a never-ending cycle of restoration and almost inevitable re-treatment, particularly in, but certainly not limited to, underserved and undereducated populations.
Dental caries is a complex, multifactorial, infectious, and chronic disease process1 caused when the byproducts of bacteria diffuse into tooth enamel and dentin and dissolve the mineral.14 Caries occurs as a result of a continuum of cyclic demineralization and remineralization of enamel.15 The formation of plaque biofilm enables the proliferation of acidogenic, aciduric, and cariogenic bacteria1,16-19 and consequently reduces salivary pH.20 Frequent consumption of dietary sugars and acidic drinks21 throughout the day further prolongs periods of low pH.1 Such extended periods cause an overgrowth of pathogenic oral bacteria, which produces acid. This in turn leads to a loss of mineral content in the tooth and caries progression into the tooth.1,20,22,23 White spot lesions develop when acids have diffused into and begun to demineralize the subsurface enamel. This is often associated with areas of plaque accumulation around orthodontic brackets. If the demineralization process is not stopped, the intact enamel surface eventually collapses and cavitates.13 The acquired pellicle has been shown to delay the progress of remineralization.24 In theory, managing the oral pH and concentrating on acid-producing microorganisms and fermentable carbohydrates might be a logical step in controlling the demineralization process; however, recent research has shown eliminating specific microorganisms may not be advisable because they are members of an endogenous microflora.24
With most people retaining many more teeth throughout their lifetimes than at any point in history, the fact that dental disease remains so prevalent may be attributable to a combination of insufficient knowledge and education, patient apathy, dietary changes, poor access to care, and difficulty in diagnosing early caries (especially interproximally).1,25 As a result of the research on plaque biofilm and the oral–systemic health connection, it is increasingly evident that dentists must understand a myriad of factors that impact oral health and caries susceptibility. These include the microbial, behavioral, environmental, and socioeconomic factors that contribute to dental caries as a disease, the patient's perception of the disease's importance, and the dental professional's role in prevention, detection, and treatment.1 Caries proliferation can be characterized by disease indicators and risk factors as well as protective factors.1,26 Dental professionals must recognize and accept that the process of caries diagnosis today is more complicated and involves thorough evaluation of known disease indicators and risk factors, a caries risk assessment, microbial measurements, radiographic evidence, and knowledge of the patient's medical and oral health histories.
Risk Factors
Caries risk factors include evidence of visible plaque and cavitations, bleeding gingiva, interproximal lesions, white spot lesions, deep occlusal pits and fissures, inadequate saliva flow, current decay conditions, bacterial challenge, oral appliances, dental implants, poor oral hygiene, lack of professional care, certain over-the-counter (OTC) and prescription medications, and dietary habits (including acidic beverage intake and frequency of snacking). Guidelines and definitions of risk categories are available from several resources, including the American Dental Association (ADA) Council on Scientific Affairs.1,27
Salivary components accelerate the remineralization process and retard demineralization.24 Normal salivary function provides antimicrobial protection, lubricates the mucosa, maintains pH, and preserves the integrity of the dentition through its mineralizing potential. The loss or decrease of normal salivary production results in xerostomia, tooth demineralization, decreased clearance of food debris, and a shift in the mouth's bacterial population that is characterized by an increase in cariogenic organisms (ie, Streptococcus mutans and Lactobacillus) at the expense of noncariogenic bacteria.28-34 Therefore, patients who have hyposalivation are at increased risk for oral sequellae, depending on the degree of salivary loss.
Saliva quantity and quality and bacterial load can be measured and monitored chairside with current diagnostic tools, such as Saliva-Check Mutans (GC America, Alsip, IL) and CRT buffer (Ivoclar Vivadent, Inc, Amherst, NY). A chairside swab test (CariScreen, Oral BioTech, Albany, OR) tests the concentration of acid-producing bacteria and, therefore, decay risk level, present in a patient's plaque biofilm. Most of these procedures are not covered by dental insurance policies and, as a result, seem to have received limited acceptance by dental practitioners.
Changing Standards of Care
Clinicians have shifted from G.V. Black's "extension for prevention" theory and instead focus on minimally invasive techniques, removing much less hard tissue during operative procedures. In tandem, clinicians have moved toward practicing evidence-based dentistry. Now, as it has become obvious that the conventional restorative approach does little to address the caries disease process,1,35 minimum intervention dentistry has morphed into following the medical model of disease control.36,37 This involves preventing disease, maintaining health, identifying caries risk, preventing incipient caries from progressing, chemically remineralizing early lesions, and repairing rather than replacing failing restorations when possible. Understanding the science of plaque biofilm led to the caries balance theory, which refers to a balance in destructive and protective factors that affects whether demineralization or remineralization occurs and thereby influences the progression or reversal of enamel caries.26
To help establish consistency in clinical decision making, especially regarding treatment of cavitated/noncavitated lesions or the choice between remineralization and restoration, a caries risk assessment model called caries management by risk assessment (CAMBRA) was developed based on the medical model for disease management.1 CAMBRA represents a paradigm shift in dentistry because it treats dental caries as a curable and preventable infectious disease and focuses on prevention and early intervention, rather than waiting for damage to tooth structure to occur.13 CAMBRA is developing preventive, therapeutic, behavioral, and restorative clinical protocols based on individual risk assessment and customizing evidence-based treatment decisions for each patient. A 2007 consensus paper identified the main principles for CAMBRA implementation as modification of the oral flora to favor health, patient education and informed participation, remineralization of noncavitated lesions, and minimal operative intervention of cavitated lesions and defective restorations.35 Numerous resources exist for strategies to implement such guidelines into private practice.35 CAMBRA should be performed at every recall visit because the patient's risk factors may change due to aging, medication use, pregnancy, chemotherapy, and increased stress levels.13,35
CAMBRA also can be used to motivate patients, helping them recognize their integral role in caries management.38 There is a call for CAMBRA to become the standard of care in private practice, and it is part of most if not all dental school curriculums. The ADA Council on Scientific Affairs endorsed caries risk assessment in January 2009 and provides a useful form for private practitioners at www.ada.org/prof/resources.
In a recent interview, Howard E. Strassler, DMD, Professor at the University of Maryland Dental School, said one of the profession's biggest challenges is that dentists are overlooking the importance of caries risk assessment.39 Exacerbating the problem, insurance companies do not recognize the necessity for or value of a caries risk assessment-based, customized treatment plans, nor do they adequately reimburse clinicians for disease prevention strategies.39
In addition to the transformation in philosophy regarding disease management, treatment techniques and restorative material options have also changed dramatically throughout the years. Dental manufacturers continue to research and develop tools for early caries detection/diagnosis, as well as esthetic materials that can be used to restore cavitated lesions at various stages of progression. Chemical and biomedical approaches to maintaining the demineralization–remineralization balance also are being developed.
Historically, while the profession's approach to caries has transitioned from reactive to proactive, addressing the caries challenge has relied on prevention and restoration, with no intermediary means to stop lesion progression. This article presents an overview of current and novel caries treatments from prevention to early detection and remineralization to a recently introduced method, called caries infiltration, that arrests the cavitation process. The caries infiltration technique appears to be the only microinvasive treatment approach currently available for stopping caries progression while restoring incipient proximal carious lesions and smooth-surface white spot lesions. This paradigm shift from reacting to the results of caries to stopping lesion progression without restoration has been slow to evolve. As dental school curriculums and reimbursement policies change, so will the profession's approach to disease prevention.
Prevention
Numerous options exist for caries prevention, which relies more heavily on patient cooperation than any other aspect of dentistry. Preventive treatment techniques, divided into primary, secondary, and tertiary categories, include good oral hygiene (self-care and patient instruction), pit-and-fissure sealants (temporary or permanent), fluoride use (patient-applied dentifrices and rinses or professionally applied varnishes), evaluation of dietary habits (as well as guidelines for modification), and other efforts to modify biofilm and decrease the cariogenic challenge.40 The dental literature provides ample studies, case reports, technique articles, and resources for preventive strategies.
Novel approaches to caries prevention include approximal sealants, slow-release fluoride applications, various remineralization methods, chlorhexidine gels or coating,41,42 biofilm modification, reduction of the cariogenic challenge with ozone therapy and probiotics,43,44 laser treatment of enamel to increase resistance to demineralization, and the Hall technique, which is a hybrid simplified method of managing carious primary molars using preformed metal crowns cemented with no local anesthesia, caries removal, or tooth preparation.45
Despite discussion of caries vaccines for more than 20 years and reports that one may be commercially available soon, the product remains in development. No one can predict its effectiveness or whether it will be embraced by the dental profession or fall under the purview of other healthcare professionals. Emerging probiotic intervention therapies that target the biologic complexities of plaque biofilms and diseases hold promise for addressing dental caries as a "complex biosocial disease."43
Early Detection
As caries detection technology has improved, so too has the ability to detect and, in turn, address early caries. Early detection allows for minimal intervention in patients receiving routine dental care.
Traditionally, dental professionals employed visual/tactile and radiographic evidence for caries detection. However, lesions, especially interproximally, typically advance so far that by the time they are visible, they require restorative intervention.38 Consequently, caries detection has evolved from radiographic film to panoramic x-rays to digital radiography to handheld tools to software combinations that can be used to enhance images and assist with analysis and documentation of caries. Various new technologies can now be used for detecting caries at its earliest stages: quantitative light-induced fluorescence (QLF); laser fluorescence; light-emitting diode refraction and reflection; and digital fiber-optic transillumination (FOTI).38,43 Infrared (IR) laser fluorescence technology detects differences between healthy and demineralized enamel, including interproximal caries (in adults), fissure caries, and smooth-surface caries.46,47
QLF measures the degree of demineralization by revealing or measuring the fluorescence of teeth with a fiber-optic sensor.13 QLF technology, which uses an algorithm to evaluate remineralization vs lesion progression,13 can be used to monitor carious lesions over time and to motivate patients by involving them in the disease management stage, rather than only later at the restoration stage.48 FOTI detects differences in light transmission/reflection in demineralized enamel on occlusal, interproximal (adults only), and pit-and-fissure surfaces.
Emerging caries detection technologies include optical coherence tomography (OCT), which measures back-scattered near-IR light to reveal porosity caused by demineralization, and polarized Raman spectroscopy (PRS), which analyzes tooth composition, mineral content, and crystallinity. OCT and PRS are designed to be used in combination and may provide much higher sensitivity and specificity than currently exists for diagnosing areas of demineralization.49
Berg and others have cautioned that confusing caries detection with caries diagnosis can result in overdiagnosis and overtreatment.38 The results of caries detection devices must be corroborated with evidence of caries lesions obtained by other means.1,38 Note that professional prophylaxis should be performed before using most caries detection devices because organic matter on the teeth can influence readings, as can sealed surfaces and stained fissures.48-50
Remineralization
The fact that caries generally progresses slowly, coupled with advances in earlier detection, has increased the period in which dental professionals can reverse the demineralization process.6,15,51-58
A consensus statement on appropriate management of dental caries, issued in 2001 by the Consensus Development Conference on the Diagnosis and Management of Dental Caries Throughout Life, called for "improved diagnosis of early non-cavitated lesions and treatment for prevention and arrest of such lesions."24,59 Chemical remineralization halts and reverses early damage caused by demineralization before surface cavitation occurs.13 Currently, restoring proximal tooth surfaces is recommended only if a bitewing radiograph shows a solid enamel radiolucency extending from the surface through the enamel and penetrating into the dentin.13,19,60,61 Therefore, with CAMBRA, if the surface is noncavitated, other treatment options should be considered before restoration is attempted.
Fluoride
Historically, remineralization has relied on topical fluoride exposure and treatment.7,13,62 Fluoridated water (if locally available) and fluoride-containing dentifrices are the most common approaches to applying topical fluoride. Professional or prescription fluoride treatments include gels and foams (maximum of 5000 ppm), rinses (223 ppm), and varnishes (23,000 ppm). Three types of fluoride are the most commonly used and approved by the Food and Drug Administration: stannous fluoride (SnF2), sodium monofluoro-phosphate (Na2PO3F), and sodium fluoride (NaF).24 Fluoride simultaneously inhibits demineralization and enhances remineralization by making teeth more acid resistant.13 It catalyzes remineralization and is bacteriostatic or bacteriocidal, depending on the level of fluoride present. Daily use of fluoride dental rinses has been demonstrated to be a clinically effective adjunct to brushing with fluoride-containing dentifrices.24 A multitude of OTC and professional fluoride products are available.
Casein Phosphopeptide-Amorphous Calcium Phosphate
Demineralization is the result of acids depleting calcium and phosphate ions from tooth enamel; remineralization is the process of replacing these minerals. The normal remineralization process uses calcium and phosphate ions from saliva to replace minerals lost by exposure to plaque acids. Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) increases the levels of calcium and phosphate ions and enhances the remineralization process.13 These phosphopeptides are similar to those found in saliva whose function is to aid remineralization. Fluoride remineralization will not occur without sufficient amounts of calcium and phosphate, so concomitant therapy is sometimes advised. However, CPP-ACP and fluoride products should be used at different times of the day for the best benefit.13 Adding ACP as a filler in sealants and composites may help with remineralization of both enamel and dentin.20,63 ACP is converted to hydroxyapatite, which precipitates and replaces the hydroxyapatite lost due to acid.20 ACP also prevents the colonization of cariogenic bacteria on dental surfaces and may, in fact, prevent demineralization.20
CPP-ACP products include Recaldent® (Bonlac Foods Ltd, Melbourne, Australia)), MI Paste (GC America), Arm & Hammer® Age Defying toothpaste (Church & Dwight Co, Inc, Princeton, NJ), and Trident® White chewing gum (Cadbury Adams USA, LLC, Parsippany, NJ).
In the case of salivary hypofunction, calcium and phosphate are depleted and must be supplied. A recently published study of caries in patients with head and neck cancer comparing the efficacy and safety of a remineralizing toothpaste (Arm & Hammer Age Defying toothpaste) that contains soluble calcium and phosphate ions with fluoride (1100 ppm) to that of a fluoride-only toothpaste showed an increase in remineralization, especially on root surfaces.64 Caphosol® (EUSA Pharma, Oxford, UK) remineralizing rinses also were effective for both root and coronal caries prevention.65
Xylitol
One highly modifiable risk factor is dietary habits.7,13,19,66 Sugar intake throughout the day results in extended periods of low oral pH, which has been clearly demonstrated to initiate caries.13,67-70 Xylitol is a natural "wood alcohol" sugar (derived from birch) that is not metabolized by oral bacteria. Xylitol is now considered a cariostatic agent because it inhibits biofilm attachment and interferes with intracellular metabolism of bacteria.13 It has been shown that ingesting 6–10 g/day significantly reduces levels of mutans streptococci and they expend energy to expel it from the cell.13,71,72 Xylitol is available in various products including gums, lozenges, mints, sprays, rinses, pastes, and sweetener substitutes.13,71 Xylitol does not stimulate the production of insulin in people with diabetes.13 It should be noted that xylitol is not safe for dogs, resulting in hepatic failure, hypoglycemia, and seizures.
Glass Ionomers
Using glass ionomers as sealants or cavity liners has recently become an option because of their "rechargeable" fluoride-releasing property.13,73,74 This material allows electrolytes in saliva to pass through, so calcium and phosphates are replenished every time the glass ionomer surface is exposed to fluoride contained in dentifrices, rinses, etc.13,75
About 71% of all restorative therapy is performed on previously restored teeth, with secondary caries as the predominant cause.13,76 A recent study comparing the effects of resin-based sealants, fluoride-containing sealants, fluoride varnish, and a glass ionomer cement on stabilizing or reversing incipient caries concluded that glass ionomer cement was the most effective in reducing carious areas and the most efficient at inhibiting new caries lesions and demineralization of intact enamel adjacent to sites where it was placed.77
Other benefits of glass ionomer cement include being hydrophilic and self-curing, requiring no etching, and bonding well to dentin. Additionally, biofilm does not adhere to glass ionomers.13,78-80 Possible drawbacks are that glass ionomers will not adhere to a desiccated tooth surface and will wear under the forces of attrition unless they are resin reinforced.36
Caries Infiltration
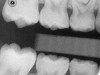
Caries infiltration is a novel technology for arresting dental caries (Icon®, DMG America, Englewood, NJ), providing a new intermediary treatment option between preventive and restorative therapies (Table 1).81 A concept developed at the Charité in Berlin, the University of Kiel, and recently introduced in the US, caries infiltration is a microinvasive approach for smooth-surface and proximal noncavitated carious lesions that avoids unnecessary loss of healthy hard tissue to operative therapy, perhaps for years (Figure 1).82
All other methods of intervention (mechanical, remineralization, or sealant therapy) require multiple visits and applications. Regardless of the technique used, restoration inevitably necessitates removing certain amounts of the healthy hard tissue surrounding a cavitated lesion. Caries infiltration can be used to arrest lesions in one patient visit with no drilling or anesthesia.
Unlike sealants applied to the surface that form a "cap" over incipient caries lesions, infiltration works by capillary action. An analogy is how a sugar cube or sponge absorbs liquid. This infiltrant has an extremely high penetration coefficient and is drawn deep within the pores of a lesion, completely filling it and stopping the diffusion of nutrients and caries progression.
Bacteria are physically too large to diffuse through an intact enamel surface.13 However, Kielbassa et al tested the accuracy of cavitation detection in proximal caries lesions at various magnifications and found that in significantly more cases than previously thought, tooth surfaces were not intact.83 Researchers have tested various adhesives and resins for penetrability through the compact layers of natural lesions and determined that after etching with hydrochloric acid to erode the "pseudointact" surface layer, low-viscosity resin infiltrants had higher penetrations and hampered lesion progression.
As of this writing, four in vitro and eight in vivo studies on caries infiltration have been completed or are continuing throughout the world. Confocal laser scanning microscopy has been used to document resin penetration depths as well as to verify lesion depths and the lack of progression after infiltration (DMG, data on file) (Figure 1).84
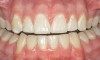
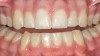
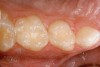
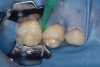
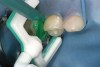
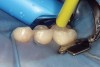
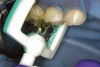
Caries infiltration is indicated for all age groups, up to the first third of dentin (D-1) (Figure 2). It is especially advantageous in interproximal areas, where a relatively large ratio of healthy hard tissue must be removed to eliminate carious tissue. Infiltration replaces hard tissue lost due to demineralization (to a maximum of 800 µm) with a low-viscosity resin, creating a barrier to further diffusion of carbohydrates and organic acids within the hard tissue, not on the tooth surface. This barrier stabilizes and effectively blocks the caries without changing the anatomic shape or appearance of the tooth.85 Additionally, treated lesions lose their whitish opaque color and blend with surrounding natural enamel, which is especially esthetic in the smooth surface type of lesions often found when fixed orthodontic appliances are removed (Figure 3 and Figure 4).
Caries infiltration is a simple, straightforward technique. Before treatment, the tooth should be thoroughly cleaned and isolated with a rubber dam (Figure 5 and Figure 6). Treatment kits contain all of the materials required for the technique (except the rubber dam), including specially designed proximal tips that are used for accurate delivery of the acid etch and infiltrant resin during the procedure. These carriers consist of an ultrathin film perforated on one side for direct placement at the treatment site, which protects adjacent teeth. The tips swivel 360°, allowing application from different angles. All syringes contained in the kit are screw-type applicators, which ensure controlled extrusion of the materials.
Specially designed dental wedges are inserted to slightly separate the carious tooth from adjacent teeth. A 15% hydrochloric gel is used to remove the "pseudointact" surface and open the pore system of the incipient lesion body (Figure 7).85 After rinsing (Figure 8), the area is dried with ethanol (Figure 9), followed by dry air. Then, the infiltrant is applied and allowed to penetrate the lesion pores by capillary action for 3 minutes (Figure 10). Any excess material is removed with dental floss, and the infiltrant is light cured from three angles for 40 seconds (Figure 11). A second layer of infiltrant is applied for 1 minute, and light cured for 40 seconds (Figure 12).86 It should be noted that the infiltrant is not radiopaque because fillers would affect the viscosity. Efficacy of the treatment can be tracked at future visits by lack of lesion progression.
Conclusion
Dental caries treatment has changed dramatically in recent years, from drilling and filling to prevention and, now, to arresting carious lesion development. Being able to stop the progression of a carious lesion on discovery—rather than watching and waiting until it has cavitated and requires restoration—is a novel concept. Caries infiltration represents a new approach to managing interproximal and smooth-surface noncavitated carious lesions with a technique that preserves tooth structure, which is the goal of dental professionals. It provides an alternative to the more invasive restorations typically used for hard-to-access interproximal and white spot lesions, especially in the esthetic zones. Additionally, because caries infiltration can be used in both primary and permanent teeth and accomplished in one visit, it should appeal to parents of young children and adolescents. As this is a new technology, the authors look forward to seeing more research and case studies using this innovative approach to caries management.
Acknowledgment
Clinical photographs courtesy of Dr. Simon Lin.
Disclosure
The authors have received an honorarium from DMG America.
References
1. Kutsch VK. Dental caries: a new look at an old disease. Inside Dentistry. 2009;5(5):60-65.
2. Preventing Dental Caries with Communities Programs. Centers for Disease Control and Prevention Web site. www.cdc.gov/
NCCdphp/publications/factsheets/Prevention/oh.htm. Accessed August 2009.
3. Griffin SO, Griffin PM, Swann JL, et al. Estimating rates of new root caries in older adults. J Dent Res. 2004;83(8):634-638.
4. García-Godoy F, Hicks MJ. Maintaining the integrity of the enamel surface: the role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J Am Dent Assoc. 2008;139(suppl):25S-34S.
5. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51-59.
6. Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol. 1999;27(1):31-40.
7. Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(7):887-899.
8. ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57(6):325-329.
9. ten Cate JM, van Loveren C. Fluoride mechanisms. Dent Clin North Am. 1999;43(4):713-742.
10. Featherstone JD. Caries prevention and reversal based on the caries balance. Pediatr Dent. 2006;28(2):128-132; discussion 192-198.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J Clin Pediatr Dent. 2003;28(1):47-52.
12. Sgan-Cohen HD. Oral hygiene improvement: a pragmatic approach based upon risk and motivation levels. BMC Oral Health. 2008;8:31.
13. Young DA, Kutsch VK, Whitehouse J. A clinician's guide to CAMBRA: a simple approach. Compend Contin Educ Dent. 2009;30(2):92-105.
14. Featherstone JD. Dental caries: a dynamic disease process. Aust Dent J. 2008;53(3):286-291.
15. Diefenderfer KE, Stahl J. Caries remineralization therapy: implications for dental readiness. Mil Med. 2008;173(suppl 1):48-50.
16. Jenson L, Budenz AW, Featherstone JD, et al. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc. 2007;35(10):714-723.
17. Penning C, van Amerongen JP, Seef RE, et al. Validity of probing for fissure caries diagnosis. Caries Res. 1992;26(6):445-449.
18. Lussi A. Validity of diagnostic and treatment decisions of fissure caries. Caries Res. 1991;25(4):296-303.
19. Fontana M, Zero DT. Assessing patients' caries risk. J Am Dent Assoc. 2006;137(9):1231-1239.
20. Sharma S, Kugel G. Amorphous calcium phosphate sealants—the potential to remineralize. Inside Dentistry. 2009;5(5):78-80.
21. Bartlett DW, Bureau GP, Anggiansah A. Evaluation of the pH of a new carbonated soft drink beverage: an in vivo investigation. J Prosthodont. 2003;12(1):21-25.
22. Oong EM, Griffin SO, Kohn WG, et al. The effect of dental sealants on bacteria levels in caries lesions: a review of the evidence. J Am Dent Assoc. 2008;139(3):271-278.
23. Beauchamp J, Caufield PW, Crall JJ, et al. Evidence-based clinical recommendations for the use of pit-and-fissure sealants: a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139(3):257-268.
24. Zero DT. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health. 2006;6(suppl 1):S9.
25. Ekstrand KR, Ricketts DN, Longbottom C, et al. Visual and tactile assessment of arrested initial enamel carious lesions: an in vivo pilot study. Caries Res. 2005;39(3):173-177.
26. Featherstone JD. The caries balance: the basis for caries management by risk assessment. Oral Health Prev Dent. 2004;2
(suppl 1):259-264.
27. American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137(8):1151-1159.
28. Vissink A, Panders AK, Gravenmade EJ, et al. The causes and consequences of hyposalivation. Ear Nose Throat J. 1988;67
(3):166-168,173-176.
29. Garg AK, Malo M. Manifestations and treatment of xerostomia and associated oral effects secondary to head and neck radiation therapy. J Am Dent Assoc. 1997;128(8):1128-1133.
30. Kielbassa AM, Hinkelbein W, Hellwig E, et al. Radiation-related damage to dentition. Lancet Oncol. 2006;7(4):326-335.
31. Harrison JS, Dale RA, Haveman CW, et al. Oral complications in radiation therapy. Gen Dent. 2003;51(6):552-560.
32. Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. J Oral Rehabil. 2007;34(10):711-723.
33. Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72(9):841-846.
34. Ooshima T, Yoshida T, Hashida T, et al. Effects of hyposalivation on the oral microflora of rats fed sucrose or wheat flour diets. Caries Res. 1992;26(2):124-131.
35. Young DA, Featherstone J, Roth JR, et al. Caries management by risk assessment: implementation guidelines. J Calif Dent Assoc. 2007;35(11):799-805.
36. Fitzgerel W, Gutkowski S. The evolution of dentistry. Inside Dental Hygiene. 2007;7(7). http://www.insidedentalhygiene.com/
print.php?id=1784. Accessed August 7, 2009.
37. World Congress of Minimally Invasive Dentistry Web site. http://www.wcmid.com/about.html. Accessed June 11, 2007.
38. Berg JH. Minimal intervention: motivating patients through caries risk assessment. Compend Contin Educ Dent. 2007;28(3):162-164.
39. DiMatteo AM. Under construction: the state of dental caries. Inside Dentistry. 2009;4(5):92-98.
40. Longbottom C, Ekstrand K, Zero D. Traditional preventive treatment options. Monogr Oral Sci. 2009;21:149-155.
41. Ersin NK, Edin E, Eronat N, et al. Effectiveness of 2-year application of school-based chlorhexidine varnish, sodium fluoride gel, and dental health education programs in high-risk adolescents. Quintessence Int. 2008;39(2):46-51.
42. Banting D, Papas AS, Clark, DC, et al. The effectiveness of 10% chlorhexidine varnish treatment on dental caries incidence in adults with dry mouth. Gerontology. 2000;17(2):67-76.
43. Duffin S. Managing caries in the high-risk child. Compend Contin Educ Dent. 2009;30(2):106-117.
44. Caglar E, Kavaloglu O, Kuscu O, et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Invest. 2007;11(4):425-429.
45. Longbottom C, Ekstrand K, Zero D, et al. Novel preventive treatment options. Monogr Oral Sci. 2009;21:156-163.
46. Ferreira JMS, Silva MFA, Oliveira AFB, et al. Evaluation of different methods for monitoring incipient carious lesions in smooth surfaces under fluoride varnish therapy. Int J Paediatr Dent. 2008;18(4):300-305.
47. Shi XQ, Tranaeus S, Angmar-Månsson B. Validation of DIAGNOdent for quantification of smooth-surfaces caries: an in vitro study. Acta Odontol Scan. 2001;59(2):74-78.
48. Heinrich-Weltzien R, Kühnisch J, van der Veen M, et al. Quantitative light-induced fluorescence (QLF)—a potential method for the dental practitioner. Quintessence Int. 2003;34(3):181-188.
49. Choo-Smith LP, Dong CCS, Cleghorn B, et al. Shedding new light on early cavities detection. J Can Dent Assoc. 2008;74(10):
913-918.
50. Lussi A, Reich E. The influence of toothpastes and prophylaxis pastes on florescence measurements for caries detection in vitro. Eur J Oral Sci. 2005;113(2):141-144.
51. Shwartz M, Gröndahl HG, Pliskin JS, et al. A longitudinal analysis from bite-wing radiographs of the rate of progression of approximal carious lesions through human dental enamel. Arch Oral Biol. 1984;29(7):529-526.
52. Foster LV. Three year in vivo investigation to determine the progression of approximal primary carious lesions extending into dentine. Br Dent J. 1998;185(7):353-357.
53. ten Cate JM, Featherstone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(3):283-296.
54. ten Cate JM. Remineralization of caries lesions extending into dentin. J Dent Res. 2001;80(5):1407-1411.
55. Newbrun E. Topical fluorides in caries prevention and management: a North American perspective. J Dent Educ. 2001;65
(10):1078-1083.
56. Autio-Gold JT, Courts F. Assessing the effect of fluoride varnish on early enamel carious lesions in the primary dentition. J Am Dent Assoc. 2001;132(9):1247-1253.
57. Biesbrock AR, Faller RV, Bartizek RD, et al. Reversal of incipient and radiographic caries through the use of sodium and stannous fluoride dentifrices in a clinical trial. J Clin Dent. 1998;9(1):5-10.
58. American Dental Association Council on Access, Prevention and Interprofessional Relation. Caries diagnosis and risk assessment. A review of preventive strategies and management. J Am Dent Assoc. 1995;126(suppl 6); 1S-24S.
59. Horowitz AM. A report on the NIH Consensus Development Conference on Diagnosis and Management of Dental Caries Throughout Life. J Dent Res. 2004;83(spec no C):15-17.
60. Jenson L, Budenz AW, Featherstone JD, et al. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc. 2007;35(10):714-723.
61. Pitts NB, Rimmer PA. An in vivo comparison of radiographic and directly assessed clinical caries status of posterior approximal surfaces in primary and permanent teeth. Caries Res. 1992;26(2):146-152.
62. American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137(8):1151-1159.
63. Cochrane NJ, Saranathan S, Cai F, et al. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42(2):88-97.
64. Papas A, Russell D, Singh M, et al. Caries clinical trial of a remineralising toothpaste in radiation patients. Gerodontology. 2008;25(2):76-88.
65. Singh ML, Papas AS. Long term clinical observation of dental caries in salivary hypofunction patients using supersaturated calcium phosphate remineralizing rinse. J Clin Dent. 2009;20:87-92.
66. Burt BA, Kolker JL, Sandretto AM, et al. Dietary patterns related to caries in a low-income adult population. Caries Res. 2006;
40(6):473-480.
67. Marsh PD. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health. 2006;6(suppl 1):S14.
68. Bradshaw DJ, McKee AS, Marsh PD. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68(9):1298-1302.
69. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(pt 2):279-294.
70. Anderson M. Chlorhexidine and xylitol gum in caries prevention. Spec Care Dentist. 2003;23(5):173-176.
71. Spolsky VW, Black BP, Jenson L. Products—old, new, and emerging. J Calif Dent Assoc. 2007;35(10):724-727.
72. Milgrom P, Ly KA, Roberts MC, et al. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85(2):177-181.
73. Alex G. The use of resin-modified glass ionomer liners under composite resins: should they be used to help control microleakage. Inside Dentistry. 2005;(need info):30-33.
74. Mitra SB. In vitro fluoride release from a light-cured glass ionomer liner/base. J Dent Res. 1991;70(1):75-78.
75. Mustafa NB, Chan DCN, Titus HW, et al. Fluoride release from restorative materials after exposure to NaF. J Dent Res. 1996;75:382.
76. Fontana M, Gonz√°lez-Cabezas C. Secondary caries and restoration replacement: an unresolved problem. Compend Contin Educ Dent. 2000;21(1):15-30.
77. Trairatvorakul C, Kladkaew S, Songsiripradabboon S. Active management of incipient caries and choice of materials. J Dent Res. 2008:87(3):228-232.
78. Ekstrand K, Qvist V, Thylstrup A. Light microscope study of the effect of probing in occlusal surfaces. Caries Res. 1987;21(4):368-374.
79. DeSchepper EJ, Thrasher MR, Thumond BA. Antibacterial effects of light-cured liners. Am J Dent. 1989;2(3):74-76.
80. Scherer W, Lippman N, Kaim J, et al. Antimicrobial properties of VLC liners. J Esthet Dent. 1990;2(2):31-32.
81. Mueller J, Meyer-Lueckel H, Paris S, et al. Inhibition of lesion progression by the penetration of resins in vitro: influence of the application procedure. Oper Dent. 2006;31(3):338-345.
82. Martignon S, Meyer-Luckel H, Tellez M, et al. Modern detection, assessment and treatment of initial approximal lesions. J Dent Res. 2009;88(spec iss A): Abstract No. 1617.
83. Kielbassa AM, Paris S, Lussi A, et al. Evaluation of cavitations in proximal caries lesions at various magnification levels in vitro. J Dent. 2006;34(10):817-822.
84. Paris S, Meyer-Lueckel H, Mueller J, et al. Progression of sealed initial bovine enamel lesions under demineralizing conditions in vitro. Caries Res. 2006;40(2):124-129.
85. Meyer-Lueckel H, Paris S, Kielbassa AM. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries Res. 2007;41(3):223-230.
86. Paris S, Meyer-Lueckel H. Influence of application frequency of an infiltrant on enamel lesions. J Dent Res. 2008;87(spec iss B):1585.
About the Authors
Gerard Kugel, DMD, MS, PhD;
Professor, Associate Dean for Research, Tufts University School of Dental Medicine, Boston, Massachusetts
Peter Arsenault, DMD, MS;
Head, Operative Division, Department of Prosthodontics and Operative Dentistry, Tufts University School of Dental Medicine, Boston, Massachusetts
Athena Papas, DMD, PhD
Johansen Professor of Dental Research, Head of the Division of Public Health Research and Oral Medicine; Co-Head, Division of Geriatric Dentistry, Tufts University School of Dental Medicine, Boston, Massachusetts