You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Chronic kidney disease (CKD) is a long-term condition in which kidney function diminishes over a period of at least 3 months, as opposed to acute kidney injury, which lasts less than 3 months.1 The kidneys play numerous vital roles, such as providing waste elimination, electrolyte balance, water regulation, and hormone secretion. The functionality of kidneys is measured by the glomerular filtration rate (GFR), with a normal rate being around 100 to 120 ml/min/1.73 m².2 Common causes of CKD include hypertension and diabetes, conditions that lead to a series of pathological changes in the kidney structure that ultimately affect the filtration capacity of the kidneys.2 In both diabetes mellitus and hypertension, hardening and scarring of glomeruli, termed glomerulosclerosis, is seen, which hinders blood filtration and waste elimination.3 Over time, this can lead to the buildup of toxins like urea in the blood, causing conditions like azotemia (ie, elevated levels of nitrogen, creatinine, and other waste products in the blood). This toxic accumulation can have systemic effects, including neurological issues, cardiac problems, bone abnormalities, and electrolyte imbalances such as hyperkalemia (high potassium levels) and hypocalcemia (low calcium levels).
CKD classification considers the disease's origin, the severity of renal function impairment (measured by the estimated GFR), and urinary protein leakage (determined by the urine albumin-to-creatinine ratio).3 The disease's stage, categorized by these parameters, aligns with the progression risk to a terminal stage demanding renal transplant and, eventually, mortality. Globally, CKD affects 9.1% of people and resulted in 1.2 million deaths in 2017.3 Diagnosis of CKD primarily involves monitoring changes in GFR over time, and severe cases might require interventions like dialysis or kidney transplant. The treatment usually focuses on managing the underlying causes and alleviating the symptoms. The condition creates a vicious cycle, particularly with hypertension as both a cause and a consequence of CKD. In addition to the management of primary causes like diabetes and hypertension, other systemic diseases and certain medications or toxins can also contribute to the onset and progression of CKD.3
Periodontitis is defined as inflammation of the periodontal tissues resulting in clinical attachment loss, alveolar bone loss, and periodontal pocketing.4 The disease's pathophysiology has been characterized in terms of its key molecular pathways. Ultimately, periodontitis leads to the activation of host-derived proteinases, which facilitates the loss of marginal periodontal ligament fibers, apical migration of the junctional epithelium, and apical spread of the bacterial biofilm along the root surface.5 The initiation and progression of periodontitis, however, are dependent on dysbiotic ecological changes in the microbiome in response to nutrients from gingival inflammatory and tissue breakdown products that enrich some species and antibacterial mechanisms that attempt to contain the microbial challenge within the gingival sulcus area once inflammation has begun.5 Gingival inflammation is caused by the formation of bacterial biofilms. Recent research supports the hypothesis that some risk factors, such as smoking, impact both an array of immunoinflammatory responses and oral dysbiosis, which could account for the variety of responses and disease progression commonly seen in clinical patient care.5 Data shows that 42% of US adults who were dentate and over age 30 years were predicted to have periodontitis, with 7.8% having severe cases. The most common demographics with severe periodontitis were smokers, Mexican Americans, non-Hispanic Blacks, and adults aged 65 years or older.6
Periodontitis is also linked epidemiologically to various other systemic conditions, including cardiovascular disease, type 2 diabetes mellitus, obesity, rheumatoid arthritis, osteoporosis, respiratory infections, inflammatory bowel disease, Alzheimer's disease, non-alcoholic fatty liver disease, and specific cancers.7 From a medical and therapeutic standpoint, it is crucial to determine whether the connection between periodontitis and these coexisting disorders is merely observational or if it may involve causal relationships. In this context, one potential mechanism contributing to the independent association of periodontitis with inflammatory comorbidities could be the presence of low-grade systemic inflammation associated with periodontitis, which is a common factor in many chronic conditions. Additionally, systemic illnesses may also influence the development and disease progression of periodontitis. For example, type 2 diabetes mellitus can exacerbate periodontitis by increasing local inflammation within periodontal tissues around the teeth and negatively impacting the makeup of the oral microbiome. Systemic inflammation associated with periodontitis is believed to result from either the bloodstream transfer of microorganisms and their by-products from the gingiva (ie, bacteremias and endotoxemias) or the release of inflammatory cytokines and other host substances associated with periodontal tissue destruction into the bloodstream.7
Connecting CKD With Periodontitis
CKD adversely affects both prognosis and quality of life because of the heightened susceptibility to a range of health problems.7 These include hypertension, diabetes, atherosclerotic complications, autoimmune disorders, systemic inflammation, urinary tract infections, kidney stones, lower urinary tract blockages, recuperation from acute kidney injury, low birth weight, and the potential for drug-related toxicity.7 The host's immune system significantly influences the development of periodontal breakdown, and abnormal immune functions might explain some familial tendencies of the disease. Systemic uremia, often seen in CKD patients, adversely affects the immune system through various mechanisms, including abnormal neutrophil activity, increased oxidative stress, and hindered immune cell development and maturation, which in turn leads to dysregulated cytokine release and compromised barrier immunity.3 This scenario contributes to an ongoing, uncontrollable, and maladaptive inflammation in individuals with CKD, putting them in a state of simultaneous immunosuppression and systemic inflammation, which can elevate risks of infection and chronic cardiovascular issues.3
Observed Similarities
In patients with periodontitis, the migration of neutrophils into gingival tissues is a crucial factor in countering bacterial invasion and maintaining gingival health. Conditions like leukocyte adhesion deficiency-1 disease, which hampers neutrophil migration, are known to trigger aggressive, early-onset periodontal breakdown. There are observed similarities in neutrophil function alterations in CKD patients, with a noted inhibition of neutrophil recruitment into inflamed tissues due to the presence of fibroblast growth factor-23.3 These similarities demonstrate a potential parallel in defective immune responses contributing to periodontal breakdown in both scenarios, ie, periodontitis and CKD. CKD patients often exhibit systemic low-grade inflammation even in the early stages, with elevated levels of serum pro-inflammatory mediators and pro-inflammatory adipokines like leptin and visfatin.3 In the broader adult population, periodontitis has been associated with higher visfatin levels in gingival crevicular fluid, correlating with the extent of periodontal breakdown and increased levels of specific bacteria in subgingival biofilm. Hence, the elevated concentrations of pro-inflammatory adipokines seen in CKD could provide another connecting factor between CKD and periodontal conditions.
Periodontitis and CKD are associated with common comorbidities. Research has demonstrated significant associations between periodontitis and systemic conditions, including cardiovascular disease, diabetes mellitus, osteoarthritis, and metabolic syndrome, among others, all of which also share a link with CKD.3,8,9,10 This overlapping association makes it challenging to ascertain the extent to which the progression of periodontitis or CKD is either a driver or a result of systemic inflammation, or how much of this inflammation is caused by other underlying conditions.
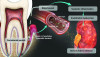
Figure 1 demonstrates the potential biologic mechanisms for periodontitis to impact local and systemic inflammation in CKD patients.
A number of cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-8, and IL-17, have been related to kidney disease and periodontitis.11 In particular, the IL-1 gene encodes inflammatory mediators that are important in the pathophysiology of both diseases. TNF-α and IL-6 plasma concentrations have been found to be sensitive indicators of odontogenic inflammation in patients with CKD.11 Additionally, a study found that hemodialysis patients' gingival crevicular fluid included significantly greater amounts of TNF-α and IL-8 than that of healthy persons, suggesting a positive link between these cytokines and clinical markers.12 By activating neutrophils and peripheral blood mononuclear cells with periodontal pathogens and specific pathogenic bacteria, respectively, these cytokines can be produced. Furthermore, long-term inflammation in periodontal tissues can activate Th17 cells to generate IL-17, which shows up in serum at higher concentrations in hemodialysis patients compared to healthy individuals.12 Moreover, it has been observed that cytokines such as IL-6 and IL-8 intensify renal inflammation, whereas TNF-α promotes pro-inflammatory effects that aid in the advancement of renal insufficiency.11
Bacteriologic Factors
Patients with CKD may experience changes in salivary flow rate and composition, which can impact the oral microbiota and contribute to periodontitis.3 Studies have noted aberrant oral bacterial communities in patients with CKD.13 A recent investigation identified a correlation between CKD and lower amounts of bacteria linked to health, such as Veillonella and Streptococcus, and higher amounts of the Gram-negative taxa Neisseria.13 Higher blood levels of pro-inflammatory cytokines, such as IL-18, were related to this shift.13 In another study, when compared to controls without renal dysfunction, patients receiving in-center hemodialysis showed a decrease in taxa linked with health, such as Rothia, and an increase in taxa associated with disease, such as Neisseria and Porphyromonas gingivalis.14 It should be noted that while this study indicated that the length of hemodialysis impacted microbial community diversity, no significant differences were noted in subgingival microbiota between patients with end-stage CKD and non-renal controls with periodontitis.15 Overall, these results point to a substantial correlation between the development of periodontitis in the setting of uremia and bacterial dysbiosis.3
The flow velocity and chemical makeup of saliva have a major impact on the oral microenvironment.3 Saliva production has been noted to be lower in people with CKD than in people with normal renal function. Along with higher concentrations of electrolytes like sodium, potassium, and phosphate and nitrogenous waste products like urea and creatinine, this decreased saliva production in CKD patients may also point to a general rise in osmolality that is correlated with the decreased flow rate.3 These salivary alterations may protect CKD patients against dental caries, but they may also increase their risk of periodontal disease.3 Additionally, in patients with CKD, marked uremia-characterized by high blood urea levels-is a common finding. Increased urea levels in gingival crevicular fluid and a noticeable elevation in salivary pH are symptoms of uremia.3 Certain periodontal pathogens such as Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum may have an ecological advantage in the periodontal pockets of patients with CKD or end-stage renal disease due to the alkaline pH of the oral cavity.3 Higher salivary urea levels in patients with severe periodontal disintegration provide indirect support for this notion.3
A dose-dependent relationship between the degree of renal dysfunction and dental calculus formation has been noted.16 This may be caused by the urea secreted by the salivary glands, which, when bacterially broken down, releases ammonia, which raises the pH of dental plaque and encourages the precipitation of calcium and phosphorus. Retention of urea seems to play a role in the alkalinization of dental plaque, which increases the production of calculus in people on dialysis. Magnesium is known to hinder the calcification process, and dialysis patients had the lowest levels of magnesium in their saliva. This might also be a factor in dialyzed patients' increased dental calculus development. Finally, a substantial portion of dental calculus in dialysis patients was caused by oxalate, which builds up in uremic situations.16,17
The term "chronic kidney disease-mineral and bone disorder" (CKD-MBD) refers to skeletal problems that CKD patients have as a result of reduced renal function.3 Low calcium and high phosphate levels in the body are the result of the kidneys' incapacity to convert vitamin D into its active form and problems controlling phosphate levels.3 Hormonal alterations ensue from this, such as increased fibroblast growth factor-23 and parathyroid hormone, which cause abnormalities in the bones, particularly high-turnover bone disease.1 Maintaining target levels of calcium, phosphate, vitamin D, and parathyroid hormone is key to managing CKD-MBD.3 Dietary calcium supplementation may assist in preventing periodontitis-related alveolar bone loss and may even contribute to slowing the disease's development and/or progression, according to certain animal studies.10
Impact of Periodontal Disease on CKD
In the gingival crevicular fluid, local periodontitis disease activity affects the amounts of acute phase proteins (APPs), such as C-reactive protein (CRP), pentraxins family protein, fibrin, and haptoglobin.11 The body produces proteolytic enzymes as the concentration of APP in plasma increases. This worsens kidney disease by causing damage to the endothelium cells in the kidney, increasing their permeability, and impairing glomerular filtration. Elevated systemic markers of inflammation, including CRP, have been associated with the presence of periodontitis, and periodontal therapy has been shown to lower serum CRP levels.18,19 By binding to C1q and complement components, CRP can activate the classical and alternative complement pathways. It can also cause thrombus development by stimulating white blood cells, releasing cytokines, and aggregating monocytes and platelets. Furthermore, CRP causes endothelial cell destruction in the kidneys and promotes the development of atherosclerotic plaque in arteries, which obstructs the renal arteries, impairs blood flow, and exacerbates kidney disease.20 This may be one mechanism explaining the impact of periodontitis on CKD stage and outcomes.
Matrix metalloproteinases (MMPs) are zinc-containing, calcium-dependent proteolytic enzymes that are essential for organ development, healthy tissue turnover, and tissue degradation.10 One such enzyme is MMP8, which is located in the periodontium, and over-activity of MMP8 is associated with excessive collagen breakdown and periodontal disease progression. MMP8 is mostly produced by neutrophils. Red complex bacteria such as Porphyromonas gingivalis, Treponema denticola, and Fusobacterium nucleatum cause periodontal inflammation. This inflammation prompts immune cells to produce MMP8 into the circulation, which is linked to systemic disorders such as CKD.21 In patients with advanced periodontitis, studies have reported greater serum MMP8 levels.22 MMPs are also involved in a variety of kidney illnesses, including renal fibrosis, diabetic nephropathy, polycystic kidney disease, and glomerulonephritis.10 They also maintain the extracellular matrix protein scaffolding. Specifically, MMP8 is detected in the urine of patients with diabetic nephropathy and is thought to drive tissue deterioration through its targeting of protease inhibitors (serpins) and collagen.23 Although there is substantial evidence linking MMPs to kidney illness and periodontitis, further research is needed to support the theory that MMPs act as a bridge between renal disease and periodontitis.
Effects of Oxidative Stress
Reactive oxygen species (ROS) buildup is a sign of oxidative stress, an imbalance between the pro-oxidative and antioxidative systems that is connected to a number of inflammatory illnesses, including periodontitis.11 Research has demonstrated that periodontitis-induced oxidative stress has a deleterious effect on the kidneys, leading to changes in renal tissue and encouraging lipid peroxidation.24,25 Further, it has been discovered that antioxidants, such as melatonin, mitigate this oxidative stress and periodontal inflammation, safeguarding the activities of the liver and kidneys.11 Studies have indicated that the presence of periodontitis increases the levels of oxidative stress and circulating ROS.11 In comparison to normal dental pulp, periodontitis-affected teeth exhibited higher levels of oxidative stress, apoptosis, and autophagy.11 Patients with periodontitis were found to have elevated levels of lipid peroxidation indicators, including as malondialdehyde. In particular, these patients' gingival crevicular fluid showed a notable level of ROS activity. Furthermore, pro-inflammatory cytokines and oxidative indicators such as 8-hydroxydeoxyguanosine (8-OHdG), a trustworthy marker of tissue oxidative damage, are elevated systemically as a result of periodontal inflammation.11 This increased oxidative stress can impair the repair and enhance the destruction of periodontal and renal tissues.
Additionally, in response to oral dysbiosis, the host response in individuals with periodontitis triggers immune cells such as polymorphonuclear leukocytes to release ROS.11 But through intricate interactions with periodontal pathogenic bacteria, this immune response, particularly the neutrophil ROS generation, induced by some pathogenic bacteria that cause periodontitis also results in local tissue damage and potentially renal tissue damage.11 One important mechanism via which periodontitis damages the kidneys may be the immunological response, specifically the generation of ROS. This link implies that reducing oxidative stress possibly with the help of antioxidants might be essential to lessening the damaging effects of periodontitis on kidney health.
Clinical Relevance and Treatments
To reiterate, periodontitis is an inflammatory condition of the gingiva and supporting structures of the teeth. Chronic inflammation in the body, as seen in periodontitis, can have a negative impact on systemic health. For individuals with CKD, who already have a compromised immune system and increased systemic inflammation, managing periodontitis becomes crucial to prevent further complications.
Some studies suggest a bidirectional relationship between periodontitis and CKD.26,27 While periodontitis may contribute to worsening kidney function, CKD itself may also increase the risk of periodontal disease. This makes it essential to address periodontitis in CKD patients to potentially slow the progression of both conditions. Both CKD and periodontitis are associated with an increased risk of cardiovascular disease. Treating periodontitis may help reduce this risk in individuals with CKD, as improved oral health can have a positive impact on overall cardiovascular health. CKD patients often have weakened immune systems, making them more susceptible to infections. Proper periodontal therapy can help control and prevent such infections. Individuals with CKD already face various health challenges and may be on complex treatment regimens. Periodontitis can cause pain, discomfort, and difficulty eating, which can further reduce the quality of life for CKD patients. Treating periodontitis can help improve oral comfort and overall well-being.
Importance of Oral Hygiene
Numerous research studies have shown that individuals with kidney disease, including hemodialysis patients and kidney transplant recipients, tend to have poor dental hygiene practices.11,28 A large-scale study revealed that regular toothbrushing can significantly reduce adverse kidney outcomes in individuals with compromised kidney function.28 In hemodialysis patients, inadequate oral hygiene has been linked to a higher risk of mortality.28 Patients undergoing dialysis, particularly those receiving hemodialysis and continuous ambulatory peritoneal dialysis, often experience a high frequency and severity of periodontitis. Managing CKD effectively involves addressing periodontitis through surgical or nonsurgical periodontal therapy, which shows promise in improving kidney function.11 This is particularly relevant when managing CKD before it reaches end-stage renal disease.11
Because of their compromised immune systems, however, CKD patients who are not receiving dialysis may not respond as well to periodontal therapy. To properly manage patients with CKD, optimal cooperation between dental and medical specialists is recommended. This is because periodontitis, which can have a major impact on renal and general health, is frequently overlooked in the face of more serious illnesses. The complicated association between periodontitis and CKD, as well as the possible advantages of periodontal therapy in this patient population, require greater study and clinical attention. Nevertheless, periodontitis knowledge among CKD patients remains poor. Inflammation in the gingival tissues causes pockets to form and bone structure to be lost as a result of periodontal disease. This process can eventually lead to tooth loss as well as additional harm to the bone and connective tissues. In addition to bacterial components, this complex interaction includes host, environmental, and behavioral influences. In conjunction with periodontitis, this condition could be called dysbiosis. From a statistical standpoint, a significant association exists between periodontitis and heightened mortality rates among individuals diagnosed with CKD. Those with CKD face an exceptionally elevated risk of mortality, with chronic inflammation emerging as a recognized contributing factor. Moreover, it is important to note that periodontitis represents one of the origins of systemic inflammation in CKD patients.11 A significant link exists between periodontitis and elevated mortality rates in individuals who have CKD.29
Irreversible loss of functioning nephrons is the hallmark of CKD, which raises the risk of heart attacks, strokes, and other neurological and cardiovascular conditions such as coronary artery calcification and hypertension. Moreover, CKD patients' defenses against viral and bacterial infections are compromised.30 CKD can be treated through several procedures and therapies such as dialysis and kidney transplant. Kidney transplantation is a replacement therapy used for patients with end-stage renal illness. Although kidney transplantation almost completely recovers kidney function, immunosuppressive therapy is required to maintain kidney graft function and avoid rejection.30 However, because of the immunosuppressive medication needed to preserve the kidney transplant's functionality, these patients are more susceptible to infections. As a result, there are disadvantages to both CKD and kidney transplantation in terms of immune system suppression.30 Inadequate oral hygiene exacerbates periodontitis.
Proactive Approach
Regular oral and periodontal examinations should be recommended for individuals with CKD. A proactive approach regarding oral hygiene can facilitate early detection and treatment of periodontitis, given its high prevalence and potential serious consequences. Identifying patient populations at the highest risk of periodontal problems can guide strategic periodontal therapy. As stated earlier, CKD remains a significant global cause of mortality and cardiovascular complications, and managing it is challenging despite advancements in early detection. It is, therefore, crucial to identify modifiable risk factors, including periodontitis, which is cost-effective to diagnose and treat. Incorporating periodontal screening through dentist referrals into the multidisciplinary management strategy for CKD patients and those at risk may prove beneficial.
Conclusion
The presence of CKD may be correlated with the existence and severity of periodontal disease. While a variety of bacteria can cause periodontal disease, some like Gram-negative bacilli are linked to the beginning and progression of CKD, especially in people with compromised immune systems. Periodontitis is a chronic disease initiated by dysbiotic biofilm, which triggers a host of inflammatory reaction, resulting in irreversible tissue damage. It is characterized by a microbial-associated, host-driven inflammation leading to the loss of periodontal attachment. Periodontitis has been linked to systemic disorders such as diabetes, liver problems, cardiovascular problems, chronic obstructive pulmonary disease, and various malignancies. The common inflammatory burden and systemic disease comorbidities may lead to a bidirectional relationship between periodontitis and CKD, and the presence and severity of periodontitis has been linked to increased severity of CKD.31
Oral health is frequently subpar among individuals with CKD, and this can contribute to issues such as protein-energy wasting, inflammation, infections, and atherosclerotic complications.32 These concerns underscore the need for ardent attention to dental care and keen awareness within clinical settings. The maintenance of a healthy and fully functional dentition in CKD patients is particularly beneficial compared to the general population. Inadequate dental health should serve as an early warning sign, even in the initial stages of CKD, among those on dialysis or preparing for kidney transplantation. In addition to oral hygiene practices, mechanical debridement and/or surgery can successfully treat periodontal diseases and reduce the systemic inflammatory burden.18,32 Lastly, suboptimal daily oral hygiene practices and insufficient awareness of the critical role of oral health in CKD patients appear to necessitate a joint effort involving both dentists and nephrologists.
About the Authors
Priscilla Sosa, DMD
Periodontal Resident, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
Maninder Kaur, BDS, MPH, MS
Associate Professor, Department of Periodontology, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama; Diplomate, American Board of Periodontology
Maria L. Geisinger, DDS, MS
Professor, Acting Chair, Program Director, Advanced Education in Periodontology, Department of Periodontology, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama; Diplomate, American Board of Periodontology
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Vassalotti JA, Fox CH, Becker BN. Risk factors and screening for chronic kidney disease. Adv Chronic Kidney Dis. 2010;17(3):237-245.
2. National Kidney Foundation. GFR. National Kidney Foundation website. https://www.kidney.org/kidneydisease/siemens_hcp_gfr. Accessed June 25, 2024.
3. Parsegian K, Randall D, Curtis M, Ioannidou E. Association between periodontitis and chronic kidney disease. Periodontol 2000. 2022;89(1):114-124.
4. American Academy of Periodontology. Glossary of Periodontal Terms. Periodontitis. AAP Connect website. https://members.perio.org/libraries/glossary/entry?GlossaryKey=d93c420e-9322-4bdd-b01c-d545af310a5b&tab=groupdetails. Accessed June 25, 2024.
5. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition [erratum in: J Periodontol. 2018;89(12):1475]. J Periodontol.2018;89 suppl 1:S159-S172.
6. Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588.e6.
7. Hajishengallis G. Interconnection of periodontal disease and comorbidities: evidence, mechanisms, and implications. Periodontol 2000.2022;89(1):9-18.
8. Van Dyke TE, Kholy KE, Ishai A, et al. Inflammation of the periodontium associates with risk of future cardiovascular events. J Periodontol.2021;92(3):348-358.
9. Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40 suppl 14:S113-S134.
10. Allen MR, Chen NX, Gattone VH 2nd, Moe SM. Adverse mandibular bone effects associated with kidney disease are only partially corrected with bisphosphonate and/or calcium treatment. Am J Nephrol.2013;38(6):458-464.
11. Li L, Zhang YL, Liu XY, et al. Periodontitis exacerbates and promotes the progression of chronic kidney disease through oral flora, cytokines, and oxidative stress. Front Microbiol. 2021;12:656372.
12. Dag A, Firat ET, Kadiroglu AK, et al. Significance of elevated gingival crevicular fluid tumor necrosis factor-alpha and interleukin-8 levels in chronic hemodialysis patients with periodontal disease. J Periodontal Res. 2010;45(4):445-450.
13. Hu J, Iragavarapu S, Nadkarni GN, et al. Location-specific oral microbiome possesses features associated with CKD. Kidney Int Rep.2017;3(1):193-204.
14. Duan X, Chen X, Gupta M, et al. Salivary microbiome in patients undergoing hemodialysis and its associations with the duration of the dialysis. BMC Nephrol.2020;21(1):414.
15. Araújo MV, Hong BY, Fava PL, et al. End stage renal disease as a modifier of the periodontal microbiome. BMC Nephrol. 2015;16:80.
16. Davidovich E, Davidovits M, Peretz B, et al. The correlation between dental calculus and disturbed mineral metabolism in paediatric patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24(8):2439-2445.
17. Martins C, Siqueria WL, Oliveira E, et al. Dental calculus formation in children and adolescents undergoing hemodialysis. Pediatr Nephrol.2012;27(10):1961-1966.
18. Yazdi FK, Karimi N, Rasouli M, Roozbeh J. Effect of nonsurgical periodontal treatment on C-reactive protein levels in maintenance hemodialysis patients. Ren Fail.2013;35(5):711-717.
19. Pejcic A, Kesic LJ, Milasin J. C-reactive protein as a systemic marker of inflammation in periodontitis. Eur J Clin Microbiol Infect Dis.2011;30(3):407-414.
20. Melnikov IS, Kozlov SG, Saburova OS, et al. Current position on the role of monomeric C-reactive protein in vascular pathology and atherothrombosis. Curr Pharm Des. 2020;26(1):37-43.
21. Ding Y, Haapasalo M, Kerosuo E, et al. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J Clin Periodontol. 1997;24(4):237-248.
22. Nizam N, Gumus P, Pitkanen J, et al. Serum and salivary matrix metalloproteinases, neutrophil elastase, myeloperoxidase in patients with chronic or aggressive periodontitis. Inflammation. 2014;37(5):1771-1778.
23. Nylund KM, Meurman JH, Heikkinen AM, et al. Periodontal inflammatory burden and salivary matrix metalloproteinase-8 concentration among patients with chronic kidney disease at the predialysis stage. J Periodontol. 2015;86(11):1212-1220.
24. Tsai CC, Chen HS, Chen SL, et al. Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40(5):378-384.
25. Cherian DA, Peter T, Narayanan A, et al. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J Pharm Bioallied Sci.2019;11(suppl 2):S297-S300.
26. Wahid A, Chaudhry S, Ehsan A, et al. Bidirectional relationship between chronic kidney disease and periodontal disease. Pak J Med Sci.2013;29(1):211-215.
27. Fisher MA, Taylor GW, West BT, McCarthy ET. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int. 2011;79(3):347-355.
28. Hirano K, Shimbo T, Komatsu Y, Kobayashi D. Frequency of tooth brushing as a predictive factor for future kidney function decline. J Nephrol. 2022;35(1):191-199.
29. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
30. Kitamura M, Mochizuki Y, Miyata Y, et al. Pathological characteristics of periodontal disease in patients with chronic kidney disease and kidney transplantation. Int J Mol Sci. 2019;20(14):3413.
31. Valenzuela-Narváez RV, Valenzuela-Narváez DR, Valenzuela-Narváez DAO, et al. Periodontal disease as a predictor of chronic kidney disease (CKD) stage in older adults. J Int Med Res.2021;49(7):3000605211033266.
32. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol.2011;6(1):218-226.