You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Root resorption is defined as the odontoclastic destruction of dental hard tissues not immediately caused by caries or trauma following the loss of the acellular protective layers of the cementum or dentin. Physiologically, resorption occurs during the exfoliation of primary teeth as a result of the pressure created by the erupting succedaneous teeth. However, resorption is considered to be pathologic in permanent teeth.1-3 Individual resorptive dental diseases may be differentiated by their tissues of origin. Internal root resorption is initiated by pulp tissue and develops in the root canal space.1,2 Oftentimes, internal root resorption occurs transiently as a tooth undergoes pulp necrosis due to severe pulpal irritants, such as caries, fractures, or the effects of trauma. The initial localized area of necrotic pulp can incite the adjacent vital tissue to resorb the root. Because the pulp is already diseased, complete necrosis usually follows swiftly; therefore, internal resorptive lesions are often only recognized once the process is arrested in an already necrotic tooth.1 The myriad presentations of external root resorption are initiated by tissues within the periodontium.1,2
Types of External Root Resorption
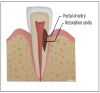
External root resorption can be further subdivided into four types: external inflammatory root resorption, replacement resorption, pressure resorption, and external cervical resorption (ECR) (Figure 1).4 Apical external inflammatory root resorption occurs secondary to pulp necrosis and the inflammatory destruction of apical bone and cementum due to the development of apical periodontitis and may be halted by endodontic treatment. Lateral external inflammatory root resorption occurs secondary to traumatic dental injuries, such as luxations or avulsions, and may be transient as long as the pulp remains vital or become progressive if pulpal inflammation leads to necrosis. Replacement resorption, otherwise known as ankylosis, is a progression of severe external inflammatory root resorption in which bone remodels in the resorptive defects. Pressure resorption occurs secondary to misaligned tooth eruption, slow-growing tumors or cysts, or orthodontic tooth movement but arrests once these stimuli are removed.4 ECR, which was historically known as invasive cervical root resorption, is a common type of external root resorption that begins at a portal of entry around the cervical region of a tooth. In ECR, cells from the junctional epithelium invade the root structure, resulting in a loss of dentin and cementum. ECR more frequently occurs in a single tooth as opposed to in multiple teeth.3-5
Etiology and Development of ECR
Although the exact etiology of ECR is unknown, several predisposing factors have been proposed.6-8 These include trauma, patient factors, iatrogenic factors, viral infections, and medical and pharmacological factors. To date, the most common potential predisposing factors to the development of ECR include orthodontics, trauma, parafunctional habits, poor oral health, and non-vital bleaching. Nonetheless, the occurrence of resorption may be idiopathic in many cases (Figure 2).1,9-12 Studies conducted in Australia, Europe, and Asia have shown varied distributions of the potential predisposing factors of ECR, which may be due to regional differences in treatment methodologies and frequencies.6,13 Recent studies also suggest that diabetes may be a risk factor for ECR because it can create a hypoxic cellular microenvironment.2,5To determine the true relationship between these predisposing factors and the development of ECR, more research is needed.
ECR is most commonly found in maxillary central incisors, followed by maxillary canines, maxillary lateral incisors, mandibular first molars, and maxillary first molars.1,13-15 Anterior teeth may be more susceptible to ECR because they experience a higher rate of traumatic injury, whereas the prevalence of ECR in posterior teeth may be a result of parafunctional habits affecting these teeth. No gender-related predisposition to ECR has been noted; however, the age distribution tends to skew toward younger patients.1
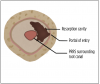
When compared with other resorptive entities, ECR lesions appear to have a unique etiology and histology. ECR lesions have three stages of development: initiation, progression, and repair-remodeling. During initiation, mechanical or chemical stress causes a disruption in the periodontal ligament that leads to a localized inflammatory response from naturally or unnaturally occurring exposed dentin and the subsequent development of granulation tissue.1,8 In the progression stage, stimulating factors activate osteoclastogenesis, which drives the resorption to further invade the tooth structure from the initial portal of entry, destroying dental hard tissues such as cementum, dentin, and enamel. ECR lesions are prevented from spreading into the pulp until late in the resorption process by the pericanalar resorption-resistant sheet (PRRS), which is also referred to as predentin (Figure 3 and Figure 4). Finally, a repair-remodeling stage may sometimes occur in parallel with lesion progression. During this stage, fibro-osseous tissue can be observed, which indicates repair by osteoblast-like cells and is responsible for osseous changes associated with ECR.1,6
Diagnosing ECR
ECR is a commonly misdiagnosed and underreported form of resorption. Understanding its unique characteristics and diagnostic presentations is essential to its recognition and appropriate management.1,14 Because ECR lesions typically present with no symptoms until late in their development when the resorption has perforated the pulp, early-stage ECR lesions are quite frequently rendered as incidental findings. Oftentimes, they are noted radiographically, particularly with the use of cone-beam computed tomography (CBCT) imaging that permits the visualization of small lesions or those presenting on the buccal or lingual surfaces of the root. ECR lesions may also be detected clinically, such as during subgingival scaling or restorative procedures.14,16 Correlating the radiographic and clinical pictures can often allow for an explorer to locate a scratchy surface or catch on the lesion. Ultimately, conducting thorough clinical and radiographic examinations is critical to identifying and diagnosing ECR.
Clinical Examination
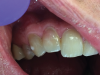
Early-stage ECR lesions may not be readily detected on clinical examination. Because the portal of entry normally occurs cervically, ECR lesions are often only palpable subgingivally. Cervical cavitation may be present and can be diagnosed by probing with an explorer-type instrument. When an explorer is used, ECR lesions will feel hard and scratchy like dentin with an irregular surface not like caries lesions, which feel sticky or soft.7,17 The appearance of knife-edge borders around such a cavitation can also help to differentiate ECR from root caries.18 Furthermore, a pink spot or banding may be present at the cervical aspect of the tooth overlying the resorption cavity; however, this finding occurs rarely and may also infrequently be associated with internal root resorption lesions (Figure 5 and Figure 6).19 The pink discoloration results because the granulation tissue of the resorptive defect has become visible through the overlying dental hard tissues. Generally, the gingiva adjacent to ECR lesions can be inflamed and may exhibit easy bleeding on probing due to its vascularity.1,7,14,19 Therefore, the presence of irregular, inflamed, and/or hyperemic gingiva may serve as early indicators of an ECR lesion. More advanced lesions may entrap debris within them, creating a secondary periodontal abscess or caries lesion with associated signs and symptoms.14,6
When the root canal has not yet been perforated by an ECR lesion, pulp sensitivity tests are commonly normal; therefore, their use is not entirely diagnostic of the entity. The lack of pulpal involvement can be explained by the PRRS, which protects the pulp from invasion until late in the resorption process.18 However, less developed ECR lesions may occasionally show signs and symptoms of reversible pulpitis due to the associated irritation and inflammation. Advanced ECR lesions that have broken through the PRRS may demonstrate signs and symptoms of irreversible pulpitis, or even necrosis, as well as the development of apical periodontitis.1
Radiographic Examination
In the detection of resorption, CBCT imaging offers improved sensitivity and specificity over periapical radiography.20-22 CBCT is able to accurately locate an ECR lesion's portal of entry, the extent of the resorption, and the proximity of the resorbing tissues to the pulp space-all of which are necessary for accurate diagnosis and management.15 Conventional radiography is limited in its ability to identify small lesions and is not as effective as CBCT when an ECR lesion is located on the buccal, lingual, or palatal walls of a tooth, which occurs in approximately 72% of cases. As a result, it is estimated that as many as 72% of ECR lesions may be missed by the use of periapical imaging alone.23,24 Nevertheless, CBCT imaging comes with the added drawback of increased radiation exposure, and it should be noted that its use should be prompted by a suspicious history as well as clinical and standard radiographic findings suggesting resorption rather than for screening purposes.22 Limited field of view CBCT is generally preferred to keep radiation dosages as low as reasonably achievable, but larger field of view CBCT scans or full mouth scans may be indicated when the presence of multiple ECR lesions is suspected.
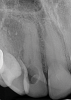
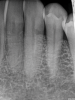
When using periapical radiographs in diagnosis, external resorptive defects should appear to move when captured from differing angulations and will have a visible and intact pulp chamber in all but advanced cases.1 All radiographic findings can help to differentiate ECR lesions from internal root resorption lesions, which have symmetrical, smooth, and clearly defined walls that appear to balloon out from the pulp chamber and do not appear to move with multiple parallax images. Conversely, ECR lesions have a less defined, irregular, and "ragged" or "moth-eaten" appearance and demonstrate variations in density, which makes them distinct from internal root resorption lesions that are usually uniform in density (Figure 7).2,14
On radiographs, early ECR lesions may exhibit a diminutive spot at the cementoenamel junction (CEJ), which may be confused with cervical burnout, but throughout the majority of the lesion, the root canal should be visible and intact. Advanced ECR lesions will appear as large radiolucent areas with less well-defined or "moth-eaten" borders that can extend into the pulp space.25 If an ECR lesion is in the repair-remodeling stage of development, it may appear more radiopaque due to the ossification of granulation tissue. Radiopaque spots may also be observed due to disruption of the PRRS that has led to local calcification of pulp tissue.6
Classifying ECR
The most commonly used classification system for ECR is the Heithersay classification, which is a 2-dimensional construction based on periapical radiography that evaluates lesions based on their extension into the root and their proximity to the root canal.1,14 A thorough breakdown of the clinical and radiographic findings associated with each Heithersay class is provided in Table 1.
Although the Heithersay classification is limited in its 2-dimensional description of ECR, the more recently developed Patel classification system developed by the European Society of Endodontology is a more detailed 3-dimensional classification system for ECR that uses CBCT imaging to describe the height of a lesion along with its circumferential spread and proximity to the root canal space (Table 2).1 As a result, the Patel classification system may better describe how ECR lesions relate spatially to the radicular structure and pulp and therefore their treatability. However, despite its comprehensive nature, as well as researchers' recommendations to utilize CBCT when available in the workup of ECR, the still-limited availability of CBCT imaging in routine dentistry has slowed adoption of the Patel classification system.
Conclusion
Early-stage ECR lesions can remain asymptomatic with limited clinical and radiographic features, making them difficult to diagnose. The accuracy of their diagnosis can be enhanced with improved clinician knowledge, a thorough patient history, and careful clinical and radiographic examination, particularly using CBCT imaging. Early detection and management can help patients retain affected teeth and improve treatment success.26 The management of ECR lesions, which requires unique considerations, will be covered in another article.
Queries regarding this course may be submitted to authorqueries@broadcastmed.com
About the Authors
Alice Li, DMD
MMSc and Certificate in Endodontics Candidate, 2026
Harvard University
School of Dental Medicine
Boston, Massachusetts
Brooke Blicher, DMD
Assistant Clinical Professor
Department of Endodontics
Tufts University
School of Dental Medicine
Boston, Massachusetts
Lecturer
Department of Restorative Dentistry and Biomaterials Science Harvard University
School of Dental Medicine
Boston, Massachusetts
Cofounder
Pulp Nonfiction Endodontics
Private Practice
White River Junction, Vermont
Rebekah Lucier Pryles, DMD
Assistant Clinical Professor
Department of Endodontics
Tufts University
School of Dental Medicine
Boston, Massachusetts
Lecturer
Department of Restorative Dentistry and Biomaterials Science Harvard University
School of Dental Medicine
Boston, Massachusetts
Cofounder
Pulp Nonfiction Endodontics
Private Practice
White River Junction, Vermont
Jarshen Lin, DDS
Director of Predoctoral Endodontics
Department of Restorative Dentistry and Biomaterials Science
Harvard University
School of Dental Medicine
Boston, Massachusetts
Clinical Associate
Department of Oral and
Maxillofacial Surgery
Massachusetts General Hospital
Boston, Massachusetts
References
1. Patel S, Mavridou, AM, Lambrechts P, Saberi N. External cervical resorption-part 1: histopathology, distribution and presentation. Int Endod J. 2018;51(11):1205-1223.
2. Lin J, Leong P, Lin LM, et al. Chapter 1: Endodontics. In: Mosby's Review for the NBDE Part II. 1st ed. Mosby; 2007:24.
3. Kim Y, Lee CY, Kim E, Roh BD. Invasive cervical resorption: treatment challenges. Restor Dent Endod. 2012;37(4):228-231.
4. Blicher B. Differentiating resorption. Communiqué. American Association of Endodontists website. https://www.aae.org/specialty/differentiating-resorption/. Published January 5, 2021. Accessed December 5, 2023.
5. Howait M, Shaker M, Aljuhani H, AlMohnna M. External cervical resorption: a case report and brief review of the literature, and treatment algorithms. J Contemp Dent Pract. 2021;22(3):298-303.
6. Chen Y, Huang Y, Deng X. A review of external cervical resorption. J Endod. 2021;47(6):883-894.
7. Talpos‑Niculescu RM, Nica LM, Popa M, et al. External cervical resorption: radiological diagnosis and literature (review). Exp Ther Med. 2021;22(4):1065.
8. Kqiku L, Ebeleseder KA, Glockner K. Treatment of invasive cervical resorption with sandwich technique using mineral trioxide aggregate: a case report. Oper Dent. 2012;37(1):98-106.
9. Chen Y, Huang Y, Deng X. External cervical resorption-a review of pathogenesis and potential predisposing factors. Int J Oral Sci. 2021;13(1):19.
10. Llavayol M, Pons M, Ballester ML, Berástegui E. Multiple cervical root resorption in a young adult female previously treated with chemotherapy: a case report. J Endod. 2019;45(3):349-353.
11. Mikušková K, Vaňuga P, Adamicová K, et al. Multiple idiopathic external cervical root resorption in patient treated continuously with denosumab: a case report. BMC Oral Health. 2022;22(1):129.
12. Arroyo-Bote S, Bucchi C, Manzanares MC. External cervical resorption: a new oral manifestation of systemic sclerosis. J Endod. 2017;43(10):1740-1743.
13. Mavridou A, Bergmans L, Barendregt D, Lambrechts P. Descriptive analysis of factors associated with external cervical resorption. J Endod. 2017;43(10):1602-1610.
14. Heithersay GS. Invasive cervical resorption: an analysis of potential predisposing factors. Quintessence Int. 1999;30(2):83-95.
15. Jeng PY, Lin LD, Chang SH, et al. Invasive cervical resorption-distribution, potential predisposing factors, and clinical characteristics. J Endod. 2020;46(4):475-482.
16. European Society of Endodontology (ESE) developed by: Patel S, Lambrechts P, Shemesh H, Mavridou A. European Society of Endodontology position statement: external cervical resorption. Int Endod J. 2018;51(12):1323-1326.
17. Patel S, Kanagasingam S, Pitt Ford T. External cervical resorption: a review. J Endod. 2009;35(5):616-625.
18. Bergmans L, Van Cleynenbreugel J, Verbeken E, et al. Cervical external root resorption in vital teeth. J Clin Periodontol. 2002;29(6):580-585.
19. Patel S, Saberi N. The ins and outs of root resorption. Br Dent J. 2018;224(9):691-699.
20. Creanga AG, Geha H, Sankar V, et al. Accuracy of digital periapical radiography and cone-beam computed tomography in detecting external root resorption. Imaging Sci Dent. 2015;45(3):153-158.
21. Durack C, Patel S. Cone beam computed tomography in endodontics. Braz Dent J. 2012;23(3):179-191.
22. Special Committee to Revise the Joint AAE/AAOMR Position Statement on use of CBCT in Endodontics. AAE and AAOMR joint position statement: use of cone beam computed tomography in endodontics 2015 update. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(4):508-512.
23. Durack C, Patel S, Davies J, et al. Diagnostic accuracy of small volume cone beam computed tomography and intraoral periapical radiography for the detection of simulated external inflammatory root resorption. Int Endod J. 2010;44(2):136-147.
24. Matny LE, Ruparel NB, Levin MD, et al. A volumetric assessment of external cervical resorption cases and its correlation to classification, treatment planning, and expected prognosis. J Endod. 2020;46(8):1052-1058.
25. Heithersay GS. Invasive cervical resorption. Endod Topics. 2004;7(1):73-92.
26. Patel J, Beddis HP. How to assess and manage external cervical resorption. Br Dent J. 2019;227(8):695-701.h