You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Successful endodontic treatment is predicated on obtaining a pretreatment pulpal and periradicular diagnosis. The pretreatment pulpal and periradicular diagnosis of a tooth begins with a review of the patient's medical and dental history. Pretreatment also includes taking a patient's blood pressure, pulse, and temperature (if indicated). If a patient presents in pain, the etiology of the pain must be identified before any emergency dental treatment is performed. The first step in determining this etiology is listening to the patient's perception of the problem, followed by a dentist's objective clinical testing to reproduce the patient's subjective pain symptoms.
If a patient presents with an asymptomatic dental condition, as often occurs in restorative dentistry, the same objective tests described below must be completed to properly make a pretreatment pulpal and periradicular diagnosis. To arrive at a proper pretreatment pulpal and periradicular diagnosis, clinicians may be uncertain of which test to perform. The following are the five objective clinical tests that a dentist must use to determine the pulpal and periradicular diagnosis.
Clinical Tests
1. Cold Test, EPT, and/or Heat Test for Pulp Sensibility
Pulp sensibility tests (thermal and electric) have been used to indirectly determine the state of pulpal health by assessing the condition of the dental pulp nerves. Pulp vitality, on the other hand, is the direct assessment of pulp blood flow.1 This assessment is obtained with laser doppler flowmetry (LDF) or pulse oximetry (PO). The reason clinicians perform sensibility tests rather than pulpal vitality tests is that LDF and PO applications in dentistry are limited (ie, they have not been designed for specific usage in dentistry).
Heat and cold tests do not jeopardize the health of the pulp.2 Additionally, teeth with porcelain or metal crowns conduct temperature and, therefore, can be tested for pulpal sensibility with cold or heat.3
With an electric pulp test (EPT), the clinician should understand what the numerical readings represent. Although the use an EPT can establish pulp sensibility, the numerical readout should not be used to determine the overall health of the pulp.4 For example, if tooth No. 8 has an EPT reading of 12 and tooth No. 9 has an EPT reading of 24, it does not mean tooth No. 8 is twice as vital as tooth No. 9. The EPT is used to determine whether the pulp is vital. In addition, when using an EPT, the clinician must be aware that teeth with metal restorations can give false-positive or false-negative responses.
Weisleder et al5 reported that the cold test and EPT used in conjunction resulted in a more accurate method for proper pulpal diagnostic testing. In another study, Jespersen et al6 reported that a pulp-testing spray and EPT are accurate and reliable methods for determining pulpal sensibility.
2. Percussion Tests for Determining the Status of the Periodontal Ligament
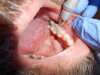
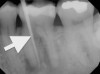
Percussion tests may be considered mistakenly to directly correlate to a pulp's sensibility. Although a tooth's sensitivity to percussion tests may be due to a pulpitis or pulpal necrosis, they are only indirectly associated. This specific test aids only in determining the status of the periodontal ligament (Figure 1). A bite test may also be necessary if a patient complains about pain while masticating.
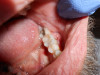
3. Palpation of the Buccal and Lingual/Palatal Gingival Tissue of the Tooth
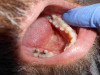
A palpation examination tests for sensitivity of the gingival tissue and for infection or inflammation of the cortical and medullary bone (Figure 2). Even when there is no radiographic evidence of an apical infection, an infection may be present clinically. A study by Bender et al7 reported it is not uncommon to have extensive disease of the bone without evidence on a radiograph.
4. Periodontal Examination Including Periodontal Probing and Tooth Mobility
Periodontal disease can develop anywhere around a tooth; therefore, the entire circumference of the tooth, or teeth, must be probed.
When evaluating tooth mobility, the clinician must remember that movement may be endodontic or periodontal in nature. In the case of periodontal disease, the tooth begins to become mobile and loosens as the attachment apparatus and surrounding bone are destroyed. With an acute endodontic infection, mobility is generally associated with an isolated tooth, but when there is generalized mobility involving multiple teeth, mobility suggests a periodontal origin.
5. Current Radiographic Examination Including Periapical, Bitewings, and/or CBCT
Uraba et al reported that cone-beam computed tomography (CBCT) imaging is effective at detecting approximately 20% more periapical lesions than are periapical radiographs, particularly in the maxillary anterior and posterior teeth.8
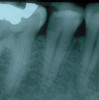
When a patient presents for restorative treatment and reports that a tooth is asymptomatic, a dentist may assume that the pulpal and periradicular diagnosis is within normal limits and hence may skip the above objective clinical tests, with the possible exception of taking a radiograph. However, using only a dental radiograph to determine the etiology of tooth pain and the pretreatment pulpal and periradicular status may lead to a pulpal and periradicular misdiagnosis (Figure 3). Therefore, a clinician must perform all five objective tests to obtain an accurate pretreatment pulpal and periradicular diagnosis.
Pulpal Diagnosis
The pulpal nerve fibers, A-delta (which respond to cold and the EPT) and C-fibers (which respond to heat and elicit the nerve response when a patient reports spontaneous tooth pain), are nociceptors. Nociceptors are sensory receptors that respond to stimuli by sending nerve signals to the brain. This stimulus can cause the perception of pain in an individual.9 By objectively testing the pulpal nerve fibers, a dentist can best determine pulpal status. Below are the current pulpal diagnosis terminologies.10
Normal pulp tests within normal limits to cold. Clinically, a patient will respond to a cold stimulus, and after the stimulus is removed, the cold sensation will dissipate immediately. The length of time it takes for a patient to respond to cold has no correlation to the diagnosis and therefore does not need to be recorded.
Reversible pulpitis is pain from an inflamed pulp that can be treated without the removal of the pulp tissue. It is not a disease, but a symptom. Classic clinical symptoms are sharp, quick pain that subsides as soon as the stimulus is removed. Physiologically, it is the A-delta fibers that are firing, not the C-fibers of the pulp.11 A-delta fibers are the myelinated, low-threshold, sharp/pricking pain nerve fibers that reside principally in the pulp-dentin junction. They can be stimulated by cold and the EPT and cannot survive in a hypoxic (low oxygen) environment. Reversible pulpitis also does not involve an unprovoked (spontaneous) response.
Symptomatic irreversible pulpitis is an inflamed pulp that cannot be treated except by the removal of the pulp tissue. Classic clinical symptoms are lingering of cold/hot stimulus greater than 5 seconds and/or patient reporting of spontaneous tooth pain. Physiologically, the A-delta fibers and/or the C-fibers can fire the neural impulses. C-fibers are the unmyelinated, high-threshold, aching-pain nerve fibers. They are distributed throughout the pulp. They are stimulated by heat and can survive in a hypoxic environment.
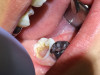
Asymptomatic irreversible pulpitis is a vital pulp that is incapable of healing, and endodontic treatment is consequently indicated. Although asymptomatic irreversible pulpitis is actually a histologic diagnosis to determine the inflammatory extent of the pulp, clinical examples of this diagnosis include a pulp polyp and internal resorption (Figure 4).
Pulpal necrosis can result from an untreated irreversible pulpitis or immediately after a traumatic injury that disrupts the vascular system of the pulp. A necrotic pulp does not respond to cold tests, EPT, or heat tests.
Previously treated: A tooth that has already been endodontically treated.
Previously initiated therapy: Endodontic treatment was started on a tooth but not completed with obturation.
Periradicular Diagnosis
When clinicians perform restorative or endodontic treatment, they do not often obtain a periradicular diagnosis. However, making a periodontal diagnosis is especially helpful when a patient presents in pain. A study by McCarthy et al12 demonstrated that patients presenting with periradicular pain can localize the painful tooth 89% of the time and that patients who present with tooth pain without periradicular pain can localize the tooth only 30% of the time.
By objectively testing the periradicular tissue, a dentist can best determine its gingival and periradicular status. Below are the current periradicular diagnosis terminologies.10
Normal periodontal tissue: Not sensitive to percussion or palpation testing. Also, radiographically, the lamina dura surrounding the root is intact.
Symptomatic apical periodontitis: The tooth has a painful response to biting and/or percussion. This may or may not be accompanied by radiographic periradicular changes.
Asymptomatic apical periodontitis: The tooth has no pain on percussion or palpation. Radiography reveals apical radiolucency.
Chronic apical abscess: Radiography typically reveals a radiolucency. Clinically, there is a sinus tract present on the gingival tissue. The draining sinus tract should be traced with a gutta-percha cone and then confirmed radiographically (Figure 5 and Figure 6).
Acute apical abscess is an inflammatory reaction to pulpal infection and necrosis characterized by rapid onset, spontaneous pain, extreme tenderness of the tooth to pressure, pus formation, and swelling of associated tissues. There may be no radiographic signs of destruction, and the patient often experiences malaise, fever, and lymphadenopathy.
Condensing osteitis is a diffuse radiopaque lesion in the periapical region. The opacity represents a localized osseous reaction to a low-grade inflammatory stimulus.
Local Anesthesia
A dentist must obtain profound anesthesia when providing endodontic treatment. A common mistake that clinicians may make when attempting to get a patient "numb" is to not objectively test whether pulpal anesthesia has been achieved before initiating endodontic treatment. Often, the only determination of whether a patient is properly anesthetized is the "subjective" anesthesia level as reported by the patient. Studies have demonstrated that inferior alveolar nerve (IAN) anesthetic blocks administered to patients with mandibular teeth diagnosed with irreversible pulpitis on average had only a 55% incidence of profound pulpal anesthesia, even in the presence of 100% lip numbness as reported by the patient.13,14
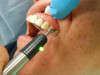
Therefore, before giving local anesthesia for endodontic treatment, the dentist should objectively test the treatment tooth with a cold test and/or EPT. With a preoperative baseline of the pulp sensibility level, after anesthesia is "onboard," the level of anesthesia can be accessed by re-testing the treatment tooth with cold or EPT (Figure 7). If the post-anesthesia tests are either negative to cold or reveal no response to EPT, there is a high likelihood that profound pulpal anesthesia has been achieved. It is important to note that teeth with metal restorations can provide a false-positive result when using the EPT. Additionally, a study by Fuss et al15 reported that in young patients, the EPT was less reliable than cold tests.
Regional and Supplemental Local Anesthesia
Another common clinical mistake that may be made when trying to achieve profound pulpal anesthesia is only giving an infiltration around the treatment tooth. This may be effective for treating a small cavity, but not for endodontic treatment. The dentist should first administer a regional block for local anesthesia. The inferior alveolar nerve block (IANB) is a regional block for the mandibular region; for maxillary teeth, a superior alveolar nerve block (SANB) is used. If profound anesthesia cannot be achieved with a regional block alone (as determined from objective testing), supplemental anesthesia should be administered. Examples of supplemental local anesthesia injections are long buccal nerve blocks (mandibular molars), periodontal ligament, intraosseous, and intrapulpal. If supplemental anesthesia is administered before a regional block, it will either be short-acting or not effective enough to provide pulpal anesthesia. In addition, re-injection of local anesthesia in the same regional or supplemental site has shown an increased success rate in achieving pulpal anesthesia.13
Choosing the Correct Local Anesthetic
In conjunction with integrating regional and supplemental local anesthesia, selecting the correct type of local anesthesia plays an important role in a clinician’s ability to obtain profound pulpal anesthesia.
Regional Block
It has been thought that the more local anesthetic containing a vasoconstrictor (ie, epinephrine) that is administered, the faster and more profound the local anesthetic effects will be for the patient. However, this methodology for administering an anesthetic with only a vasoconstrictor can actually delay the uptake of anesthetic in patients.16
Epinephrine is acidic and therefore lowers the pH of the injection site. This lowered pH will enable fewer amounts of base to be available for nerve sheath penetration. Also, although epinephrine acts on both alpha receptors (vasoconstriction) and beta receptors (vasodilatation, bronchodilation, and increased heart rate and contraction), its effect on beta receptors is equal to its effect on alpha receptors.17 Therefore, the amount of vasoconstriction that will actually occur in the injection site will be affected.
When giving a regional block, either an IANB or SANB, the dentist should administer 1 carpule of an anesthetic with a vasoconstrictor and then wait a few minutes to observe whether the patient reports any subjective signs (“feeling numb” or “feeling my lip or cheek is fat”). The first injection with an anesthetic with vasoconstrictor will help maintain the anesthetic in the region of the block.
If the patient states that he or she feels the anesthetic is taking effect, the clinician did not miss the anatomical block and should proceed with an anesthetic with no vasoconstrictor. If the patient does not report “feeling numb” or any other subjective equivalent, it is a sign that either the anatomical block was missed or there was not enough concentration of base “onboard” to penetrate the nerve sheath. Either way, the second carpule of anesthesia should consist of 3% mepivacaine with no epinephrine. The subsequent injections with 3% mepivacaine will not further lower the surrounding pH and therefore enhance the amount of base to be available to penetrate the nerve sheath. After administering the second carpule of anesthetic, if the patient begins to feel numb, it was due to anesthetic concentration; if not, the clinician must reevaluate the anatomical placement of the anesthetic.
Since the introduction of articaine into the US market, there have been studies that have demonstrated there is no significant difference between 4% articaine with 1:100,000 epinephrine and 2% lidocaine with 1:100,000 epinephrine in IANB anesthesia.18,19 It is important to note that studies have reported a higher incidence of paresthesia when articaine has been administered for IANB. Although the exact etiology of the paresthesia is unknown, it is hypothesized that the neurotoxicity may be due to the higher concentration of local anesthesia used: 4% articaine as compared with 2% lidocaine.20,21 The clinician must consider these risks along with the benefits of administering 4% articaine for IANB anesthesia.
Buccal Block
In anesthesia for a buccal block, the use of 4% articaine with 100,000 epinephrine should be the drug of choice.19 A study by Srinivasan et al22 reported that the efficacy of 4% articaine with 100,000 epinephrine was superior to 2% lidocaine with 100,000 epinephrine for buccal infiltrations in maxillary posterior teeth. Another study, by Brandt et al,18 demonstrated better pulpal anesthesia with articaine versus lidocaine when using it as an infiltration local anesthesia.
Periodontal Ligament Block
The use of 2% lidocaine with 100,000 epinephrine has been demonstrated to be significantly better in achieving pulpal anesthesia through a periodontal ligament injection than using a local anesthetic without a vasoconstrictor.23,24
Intraosseous Block
Although the literature supports the use of an anesthetic with vasoconstrictor for administering for an intraosseous block,25 the clinician needs to be aware that this will increase heart rate in most patients.26 Therefore, the clinician needs to balance a longer anesthetic effect along with a patient’s tachycardia response versus using 3% mepivacaine (with no epinephrine) and eliminating the cardiac effect but shortening the duration of the local anesthetic effect. A study by Reisman et al27 reported that when a repeated intraosseous injection with 3% mepivacaine (with no epinephrine) was administered, there was an increase in anesthetic success to 98%. Empirically, most patients get an uncomfortable feeling when their heart begins to race after the epinephrine enters their system as the result of the intraosseous block.
Intrapulpal Block
The main objective in administering an intrapulpal anesthesia is to give it under pressure. Although it has been stated in the scientific literature that saline is as effective as 2% lidocaine with 1:100,000 epinephrine in providing anesthesia intrapulpally,28,29 it is recommended to use 2% lidocaine with 1:100,000 epinephrine. Empirically, the use of anesthetic with a vasoconstrictor will provide some vasoconstriction on the pulpal vascular system. This is important because most pulps with irreversible pulpitis can be hyperemic due to the body’s attempt to address the localized inflammation.
NSAID Usage Preoperatively Before Local Anesthesia
In a recent systematic review with meta-analysis and trial sequential analysis by Nagendrababu et al,30 it was reported that a preoperative oral dosage of ibuprofen greater than 400 mg can increase the success of local anesthesia when an IANB is used on patients with irreversible pulpitis. The report also stated that ketorolac 10 mg and diclofenac 50 mg are effective alternative premedications that have shown to increase the efficacy of IANB in patients with irreversible pulpitis.
Conventional Endodontic Treatment
After proper diagnosis and profound local anesthesia, a dentist can then proceed with clinical conventional endodontic treatment in performing a pulpectomy (the complete removal of the pulp tissue) while using a rubber dam to isolate the treatment tooth. For a pulpectomy, chemomechanical preparation of the entire root-canal system should be performed. This type of chemomechanical canal preparation involves using endodontic files, sodium hypochlorite, and ethylenediaminetetraacetic acid (EDTA) gel placement on each file (Figure 8). Also, if the dentist is not doing a single-visit treatment, calcium hydroxide should be placed in the canal(s) before temporizing the tooth in cases of irreversible pulpitis.16 If the canal(s) is/are necrotic, it is recommended to also irrigate with chlorhexidine before placing a calcium hydroxide in the canal and temporizing the tooth.31
Modified Crown-Down Filing Technique
Initially, hand files should be used to access the root canal, create a glide path, and determine working length. Hand files should then enlarge the canal at working length to at least a 20/.02 to 30/.02 file size. The size will depend on the actual tooth that is being treated. After this step, rotary-file instrumentation should be initiated. It has been documented in scientific literature that rotary nickel-titanium (NiTi) files can prepare a canal faster than hand files.32
Although there are many different file techniques for conventional endodontic treatment, the modified crown-down technique is a consistent and efficient method of treatment.33 The technique involves opening the coronal two-thirds of the canal with rotary files. Several types of "orifice opener" rotary files are on the market. Next, the rotary files should be taken to working length and worked up from smaller- to larger-size files. It is recommended to use a 0.04 taper rotary-file system when preparing the root canal for obturation. The last rotary-file size that can be taken to working length in a canal is considered the master apical file.
A clinician must use a rubber dam for tooth isolation when performing endodontic treatment. Also, the occlusion should be adjusted before endodontic access on posterior teeth. This will aid in providing consistent file reference points and reducing postoperative periodontal-ligament inflammation.34
Residual Canal Debris
Residual canal debris is organic and/or inorganic material that remains on the dentinal wall after conventional endodontic chemomechanical canal preparation is completed.35 This residual canal debris is also referred to as the smear layer. The organic and/or inorganic substance is derived from ground dentin; pulpal remnants; and, in cases of infected root-canal systems, bacteria.36
A possible explanation for the residual canal debris after chemomechanical canal preparation is that NiTi rotary files remain centered in the canal and therefore will not make contact with all the dentinal walls due to various invaginations and irregularities.37 An in vitro study by Chuste-Guillot demonstrated that regardless of which NiTi rotary-file system a clinician used to prepare an infected root-canal system, the root dentin that remained was infected and not bacteria-free.38 Lin et al reported that the major factors associated with endodontic failures were the persistence of bacterial infection in the canal space and/or the periradicular area.39
Another explanation for the presence of residual canal debris after canal instrumentation and irrigation may be that a clinician is not being vigilant in using EDTA and sodium hypochlorite.40 Lastly, canal morphology can be complex, making it difficult for the chemomechanical canal preparation to be effective in removing all the canal debris.41
The three main factors in removing residual canal debris are irrigation activation, mechanical debridement, and chemical debridement. Irrigation activation with ultrasonic, a polymer finishing file, polymer ultrasonic tip, positive pressure syringe, and negative pressure device have all demonstrated various abilities in removing residual canal debris.41,42
Obturation
The clinical goal of endodontic obturation of a root-canal system is to fill empty spaces, promote hermetic sealing, and prevent bacterial activity from infiltrating the periapical tissues.43 Although gutta-percha has been a consistent material used in canal obturation for the last 40 years, techniques and sealers have changed during that time. Various techniques, such as lateral compaction, warm vertical compaction, and carrier-based obturation, have been reported in the scientific literature.44,45 In regard to sealers, the most current trend is the bioceramic sealer.46 It has been reported that bioceramic sealers are nontoxic, are hydrophilic, expand upon setting, and are antimicrobial.47
Pain Medication and Antibiotics
The most consistent predictive factor for postoperative endodontic pain is the presence of preoperative hyperalgesia (spontaneous pain, reduced pain threshold, and/or increased perception of noxious stimuli).48 A clinical study by Ali et al49 showed that postoperative pain was present in 54.5% of patients treated. A common clinical mistake in endodontic-treatment pain management is prescribing drugs after treatment without critically assessing whether the drugs are pharmacologically treating inflammation and/or infection. An example would be prescribing antibiotics for tooth pain that has an inflammatory rather than an infection etiology. Fouad50 reported that antibiotics do not have an analgesic effect on odontogenic inflammatory pain. The pretreatment endodontic and periradicular diagnosis is a clinical guide for determining inflammation and/or infection. If the treatment diagnosis is irreversible pulpitis with or without symptomatic apical periodontitis, the condition is strictly inflammation, and anti-inflammatory drugs (NSAIDs) are the medication of choice.
Significant reduction in odontogenic pain from inflammation can be seen from 400 mg to 800 mg of ibuprofen.51 A recent study by Taggar et al52 reported that ibuprofen sodium dihydrate provided faster pain relief than ibuprofen acid. In cases when ibuprofen alone is not effective in reducing postoperative pain for an endodontic patient, administering a combination of ibuprofen and acetaminophen can produce significantly effective pain management for odontogenic-type inflammation.53 Acetaminophen, alone or in combination with an opioid (eg, hydrocodone), is a good alternative analgesic for a patient who cannot take NSAID medication.35
There will be cases in which NSAIDs do not relieve a patient's odontogenic postoperative pain. Although opiate medications are commonly prescribed in these scenarios, a dentist also should consider prescribing dexamethasone, a synthetic adrenocortical steroid.54
If there is an odontogenic infection, a patient should additionally be placed on an antibiotic because steroids can block or mask the body's response to infection. When dexamethasone is prescribed, the patient should stop taking NSAIDs if they are currently being taken for pain because they can lead to an increased risk of developing stomach problems, such as a bleeding stomach ulcer.
The current trend in prescribing antibiotics in endodontic treatment is as adjunctive treatment to conventional or surgical endodontic treatment. Adjunctive antibiotic treatment may be necessary for preventing the spread of infection, in acute apical abscesses with systemic involvement, and for progressive and persistent infections.55 Systemic involvement in clinical infection can appear as fever, swelling, malaise, a compromised airway, or cellulitis, as well as in a medically compromised patient.
Penicillin V potassium (pen VK) has been documented in the scientific literature as the antibiotic of choice for endodontic infections.56 It has been demonstrated that the pen VK spectrum of antimicrobial activity includes many of the bacteria that have been isolated in endodontic infections.57 Segura-Egea et al55 reported that amoxicillin or amoxicillin with clavulanic acid showed a better absorption, higher blood levels, better tissue penetration, and fewer adverse side effects than pen VK. Amoxicillin and amoxicillin with clavulanic acid have a wider spectrum of activity than pen VK.58 This spectrum includes many species of bacteria found elsewhere in the body and may increase the risk of selecting bacteria resistant outside the oral cavity. However, amoxicillin and amoxicillin with clavulanic acid are indicated for the treatment of immunocompromised patients who may have odontogenic infections containing non-oral bacteria.57
For the patient allergic to penicillin/amoxicillin or if penicillin/amoxicillin has been ineffective, clindamycin is the second antibiotic of choice. Clindamycin is beta-lactamase resistant (unlike pen VK) and has a good spectrum against gram-positive and gram-negative bacteria.58 Another option if pen VK is ineffective is to add metronidazole along with the pen VK. Metronidazole should not be given as the sole antibiotic, but rather in combination with pen VK. Metronidazole has a narrow therapeutic spectrum against obligate anaerobic bacteria.59
When prescribing antibiotics, it is important to use a loading dose. Antibiotics with long half-lives can require several days of therapy to achieve effectiveness. In addition, the most critical time for antibiotic effectiveness is the first 24 hours, which is typically when inoculum of infection is high and likely to harbor resistant subpopulations of bacteria.60,61
Scientific literature has stated that clinicians prescribe antibiotics in courses of 3 to 7 days.62 Some evidence suggests that perhaps shorter courses (2 to 3 days) of antibiotic therapies may be as successful.63 The use of amoxicillin for 7 days has been shown to increase the population of resistant strains of bacteria.64 In addition, the dentist should be in close contact with the patient who is taking antibiotics in the event that clinical symptoms worsen or there is a drug allergy.65 Finally, if an endodontic patient presents with an intraoral fluctuant swelling, the clinician should perform an incision-and-drain procedure.66
Conclusion
Although the clinical advances and changes in thought within the field of endodontics have been significant in recent years, general dentists and associated professionals who become educated on the changes will remain up-to-date. By explaining currently accepted practices in endodontics regarding diagnosis, local anesthesia, instrumentation/obturation, and pain medications and antibiotics, this article serves as a guide for clinicians to stay updated in the field.
About the Author
James Bahcall, DMD, MS, FICD, FACD
Dr. Bahcall is Clinical Associate Professor in the Department of Endodontics, University of Illinois-Chicago College of Dentistry, Chicago, Illinois. He earned his DMD from the Tufts University School of Dental Medicine, a Certificate in Endodontics from the Marquette University School of Dentistry, and an MS from Marquette. His dental teaching career began at the Northwestern University School of Dentistry in the Department of Endodontics, where he eventually became Chair of that department. After Northwestern’s dental school closed he moved on to Marquette, where he eventually became Chair of the Department of Surgical Sciences and Director of the Endodontic Division. He later taught at the Midwestern University College of Dental Medicine before joining the UIC College of Dentistry.
References
1. Alghaithy RA, Qualtrough AJ. Pulp sensibility and vitality tests for diagnosing pulpal health in permanent teeth: a critical review. Int Endod J. 2017;50(2):135-142.
2. Rickoff B, Trowbridge H, Baker J, et al. Effects of thermal vitality tests on human dental pulp. J Endod. 1988;14(10):482-485.
3. Miller SO, Johnson JD, Allemang JD, Strother JM. Cold testing through full-coverage restorations. J Endod. 2004;30(10):695-700.
4. Lado EA, Richmond AF, Marks RG. Reliability and validity of a digital pulp tester as a test for measuring sensory perception. J Endod. 1988;14(7):352-356.
5. Weisleder R, Yamauchi S, Caplan DJ, et al. The validity of pulp testing: a clinical study. J Am Dent Assoc. 2009;140(8):1013-1017.
6. Jespersen JJ, Hellstein J, Williamson A, et al. Evaluation of dental pulp sensibility tests in a clinical setting. J Endod. 2014;40(3):351-354.
7. Bender IB, Seltzer S. Roentgenographic and direct observation of experimental lesions in bone: I. 1961. J Endod. 2003;29(11):702-706.
8. Uraba S, Ebihara A, Komatsu K, et al. Ability of cone-beam computed tomography to detect periapical lesions that were not detected by periapical radiography: a retrospective assessment according to tooth group. J Endod. 2016;42(8):1186-1190.
9. Mattscheck D, Law AS, Nixdorf DR. Diagnosis of nonodontogenic toothache. In: Hargreaves KM, Berman LH, Rotstein I, eds. Cohen's Pathways of the Pulp. 11th ed. St. Louis, MO: Mosby Elsevier; 2016.
10. Glickman GN. AAE Consensus Conference on Diagnostic Terminology: background and perspectives. J Endod. 2009;35(12):1619-1620.
11. Kim S. Neurovascular interaction in the dental pulp in health and inflammation. J Endod. 1990;16(2):48-53.
12. McCarthy PJ, McClanahan S, Hodges J, Bowles WR. Frequency of localization of the painful tooth by patients presenting for an endodontic emergency. J Endod. 2010;36(5):801-805.
13. Cohen HP, Cha BY, Spångberg LS. Endodontic anesthesia in mandibular molars: a clinical study. J Endod. 1993;19(7):370-373.
14. Nusstein J, Reader A, Nist R, et al. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1:100,000 epinephrine in irreversible pulpitis. J Endod. 1998;24(7):487-491.
15. Fuss Z, Trowbridge H, Bender IB, et al. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12(7):301-305.
16. Bahcall J. Everything I know about endodontics, I learned after dental school, part 1. Dent Today. 2003;22(5):84-89.
17. Malamed SF. Handbook of Local Anesthesia. 5th ed. St. Louis, MO: Elsevier Mosby; 2004:45-49.
18. Brandt RG, Anderson PF, McDonald NJ, et al. The pulpal anesthetic efficacy of articaine versus lidocaine in dentistry: a meta-analysis. J Am Dent Assoc. 2011;142(5):493-504.
19. Mikesell P, Nusstein J, Reader A, et al. A comparison of articaine and lidocaine for inferior alveolar nerve blocks. J Endod. 2005;31(4):265-270.
20. Haas DA, Lennon D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61(4):319-320.
21. Garisto GA, Gaffen AS, Lawrence HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141(7):836-844.
22. Srinivasan N, Kavitha M, Loganathan CS, Padmini G. Comparison of anesthetic efficacy of 4% articaine and 2% lidocaine for maxillary buccal infiltration in patients with irreversible pulpitits. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):133-136.
23. Meechan JG. Supplementary routes to local anesthesia. Int Endod J. 2002;35(11):885-896.
24. Gray RJ, Lomax AM, Rood JP. Periodontal ligament injection: with or without a vasoconstrictor? Br Dent J. 1987;162(7):263-265.
25. Repogle K, Reader A, Nist R, et al. Anesthetic efficacy of the intraosseous injection of 2% lidocaine (1:100,000 epinephrine) and 3% mepivacaine in mandibular first molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(1):30-37.
26. Coggins R, Reader A, Nist R, et al. Anesthetic efficacy of the intraosseous injection in maxillary and mandibular teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(6):634-641.
27. Reisman D, Reader A, Nist R, et al. Anesthetic efficacy of the supplemental intraosseous injection of 3% mepivacaine in irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(6):676-682.
28. Malamed SF. The management of pain and anxiety. In: Cohen S, Burns RC, eds. Pathways of the Pulp. 7th ed. St. Louis: Mosby; 1998:665-666.
29. VanGheluwe MS, Walton R. Intrapulpal injection: factors related to effectiveness. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(1):38-40.
30. Nagendrababu V, Pulikkotil S, Veettil S, et al. Effect of nonsteroidal anti-inflammatory drugs as an oral premedication on the anesthetic success of inferior alveolar nerve block in treatment of irreversible pulpitis: a systematic review with meta-analysis and trial sequential analysis. J Endod. 2018;44(6):914-922.
31. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24(7):472-476.
32. Short JA, Morgan LA, Baumgartner JC. A comparison of canal centering ability of four instrumentation techniques. J Endod. 1997;23(8):503-507.
33. Bahcall J, Johnson B. Clinical guide to treating endodontic emergencies. Inside Dent. 2016;12(4):46-51.
34. Rosenberg PA, Babick PJ, Schertzer L, Leung A. The effect of occlusal reduction on pain after endodontic instrumentation. J Endod. 1998;24(7):492-496.
35. West R, Bahcall J, Olsen K. Removing residual canal debris after rotary nickel titanium instrumentation. Endod Pract. 2008;10(2):22-24.
36. Mader CL, Baumgartner JC, Peters DD. Scanning electron microscope investigation of the smeared layer on root canal walls. J Endod. 1984;10(10):477-483.
37. Guelzow A, Stamm O, Martus P, Kielbassa AM. Comparative study of six rotary nickel-titanium systems and hand instrumentation for root canal preparation. Int Endod J. 2005;38(10):743-752.
38. Chuste-Guillot MP, Badet C, Peli JF, Perez F. Effect of three nickel-titanium rotary file techniques on infected root dentin deduction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):254-258.
39. Lin LM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18(12):625-627.
40. Bahcall J, Olsen K. Clinically enhancing the connection between endodontic and restorative treatment for better case prognosis. Dent Today. 2007;26(1):98-103.
41. Townsend C, Maki J. An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. J Endod. 2009;35(7):1040-1043.
42. Chopra S, Murray PE, Namerow KN. A scanning electron microscopic evaluation of the effectiveness of the F-file versus ultrasonic activation of a K-file to remove smear layer. J Endod. 2008;34(10):1234-1235.
43. Bueno CR, Valentim D, Marques VA, et al. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz Oral Res. 2016;30(1). doi: 10.1590/1807-3107BOR-2016.vol30.0081.
44. Leduc J, Fishelberg G. Endodontic obturation: a review. Gen Dent. 2003;51(3):232-233.
45. Da Silva D, Endal U, Reynaud A, et al. A comparative study of lateral condensation, heat-softened gutta-percha, and a modified master cone heat-softened backfilling technique. Int Endod J. 2002;35(12):1005-1011.
46. Koch K, Brave DG. A new endodontic obturation technique. Dent Today. 2006;25(5):102-107.
47. Koch KA, Brave DG, Nasseh AA. Bioceramic technology: closing the endo-restorative circle, part I. Dent Today. 2010;29(2):100-105.
48. Mattscheck DJ, Law AS, Noblett WC. Retreatment versus initial root canal treatment: factors affecting posttreatment pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(3):321-324.
49. Ali A, Olivieri JG, Duran-Sindreu F, et al. Influence of preoperative pain intensity on postoperative pain after root canal treatment: a prospective clinical study. J Dent. 2016;45:39-42.
50. Fouad AF, Rivera EM, Walton RE. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(5):590-595.
51. Torabinejad M. Cymerman JJ, Frankson M, et al. Effectiveness of various medications on postoperative pain following complete instrumentation. J Endod. 1994;20(7):345-354.
52. Taggar T, Wu D, Khan AA. A randomized clinical trial comparing 2 ibuprofen formulations in patients with acute odontogenic pain. J Endod. 2017;43(5):674-678.
53. Cooper SA. The relative efficacy of ibuprofen in dental pain. Compend Contin Educ Dent. 1986;7(8):578-581.
54. Krasner P, Jackson E. Management of posttreatment endodontic pain with oral dexamethasone: a double-blind study. Oral Surg Oral Med Oral Pathol. 1986;62(2):187-190.
55. Segura-Egea JJ, Gould K, Şen BH, et al. Antibiotics in endodontics: a review. Int Endod J. 2017;50(12):1169-1184.
56. Kuriyama T, Karasawa T, Nakagawa K, et al. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(5):600-608.
57. Baumgartner JC, Xia T. Antibiotic susceptibility of bacteria associated with endodontic abscesses. J Endod. 2003;29(1):44-47.
58. Gilmore WC, Jacobus NV, Gorbach SL, et al. A prospective double-blind evaluation of penicillin versus clindamycin in the treatment of odontogenic infections. J Oral Maxillofac Surg. 1988;46(12):1065-1070.
59. Sandor GK, Low DE, Judd PL, Davidson RJ. Antimicrobial treatment options in the management of odontogenic infections. J Can Dent Assoc.1998;64(7):508-514.
60. Drusano GL. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat Rev Microbiol. 2004;2(4):289-300.
61. Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56(6):2795-2805.
62. AAE position statement: AAE guidance on the use of systemic antibiotics in endodontics. J Endod. 2017;43(9):1409-1413.
63. Martin MV, Longman LP, Hill JB, Hardy P. Acute dentoalveolar infections: an investigation of the duration of antibiotic therapy. Br Dent J. 1997;183(4):135-137.
64. Lacey RW, Lord VL, Howson GL, et al. Double-blind study to compare the selection of antibiotic resistance by amoxicillin or cephradine in the commensal flora. Lancet. 1983;2(8349):529-532.
65. Bahcall J. Everything I know about endodontics, I learned after dental school, part 2. Dent Today. 2003;22(8):62-68.
66. Newman MG, Kornman KS. Antibiotic-Antimicrobial Use in Dental Practice. Chicago, IL: Quintessence; 1990.