You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
More than 64 million people older than 30 years have some type of periodontal disease, which is the leading cause of tooth loss. Clinicians use various physical measurements (eg, probing depth, mobility, bone loss, attachment level, recession, inflammation present) to define periodontal disease.1 Periodontitis is a chronic infection that affects the gums (gingiva) and bone that support the teeth in the maxilla and mandible.2 There are varying levels of the disease, ranging from mild gingivitis to severe periodontitis. Until about 20 years ago, it was thought and accepted by the dental profession that all individuals eventually become susceptible to periodontitis and that the continuous loss of bone and structural support eventually lead to tooth loss.3
Periodontal disease is caused by the mouth being full of bacteria. Bacteria, mucous, and other particles constantly form a sticky, colorless plaque on teeth, referred to as biofilm.4 One of the most pervasive diseases, periodontitis is characterized by the elimination of soft-tissue and bone support after an inflammatory host response secondary to an infection caused by the presence of periodontal bacteria.5 It is now generally agreed that almost all forms of periodontal disease occur as a result of mixed microbial infections within which specific groups of pathogenic bacteria coexist.6
The primary colony of bacteria consists of aerobic and facultative anaerobes such as Gram-positive cocci (eg, streptococci). Gram-positive rods appear, increase in number, and eventually outnumber the cocci. Gram-positive filaments, such as Actinomyces spp., may later outnumber all the bacteria. There are specific surface receptors on the Gram-positive cocci and rods that allow the adherence of Gram-negative bacteria, which otherwise lack the ability to attach directly to the biofilm. As time progresses, there is a shift in the microflora from Gram-positive to Gram-negative organisms and an increase in heterogeneity of the microbial species. Stable bacterial communities are established, with nutrients being exchanged between different microbes and also the production of bacteriocins, which kill specific bacteria.7
Risk Factors and Consequences of Periodontal Disease
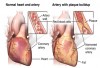
Risk factors such as smoking, poor oral hygiene, diabetes, medication, age, heredity, and stress are related to periodontal disease.7 Untreated periodontitis may increase the risk of obesity, heart attack, and other health problems. The oral cavity is a window to health. One may think there is no connection between the mouth and the heart, but periodontal disease and the heart are linked. Study authors suspect that bacteria present in gum disease can travel throughout the body, triggering inflammation in the heart's valves.8 Studies have shown that patients with periodontitis are twice as likely to have coronary artery disease as those who do not have gum disease.8 The same bacterial plaque found in patients who have periodontal disease has been found in the arteries of patients with heart disease (Figure 1). Patients with gum disease have been found to be 2.63 times more likely to have a stroke as those without gum disease.9
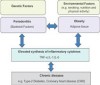
Obesity appears to be linked to periodontitis through the increased production of reactive oxygen species. Periodontal infection, which is a source of low-grade inflammation, secretes a variety of cytokines and hormones that mediate inflammation.10 Additionally, people who have deficient levels of calcium and vitamin C are predisposed to periodontal disease.11 Although the underlying pathophysiologic mechanism remains unclear, it has been demonstrated that the development of insulin resistance as a consequence of a chronic inflammatory state and oxidative stress could be implicated in the association between obesity and periodontitis.12 If periodontitis is left untreated, ongoing low-grade bacteremia is produced, adding to the cumulative inflammatory burden in the same manner as obesity. This leads to a greater risk and occurrence of inflammatory-driven diseases.13 Researchers have found increased body mass index, waist circumference, and percentage of body fat to be associated with an increased risk of developing periodontitis. These findings suggest that changes in body chemistry affect metabolism, which centers the relationship on what obesity and periodontal disease have in common: inflammation (Figure 2).10
Periodontal Health Treatment and Maintenance
Taking care of one's mouth helps an individual take care of their overall health. The success of long-term periodontal health maintenance and treatment are related to a patient's compliance with a home care protocol and timely dental visits. There are an array of products, tools, and methods for office and home care maintenance that play an important role in maintaining long-term periodontal health in patients. The tools and methods that dentists and hygienists provide at the office are unique for each patient. Maintaining good oral hygiene is imperative for healthy teeth and gingiva. Various tools and methods are suggested and used for the purpose of removing food particles and other deposits from surfaces of teeth to keep the dentition and gums healthy and disease free.10 Patients with gingivitis or periodontitis often struggle with home care between dental appointments. Lack of care can lead to severe periodontal disease and other related systemic diseases.
One treatment used for patients who have poor oral hygiene and have neglected professional cleanings in the dental office is a full-mouth debridement. A debridement consists of removing as much of the supragingival plaque and tartar as possible, which allows a thorough evaluation to be completed by the dentist. This procedure cannot be completed on the same day as a detailed and periodontal comprehensive examination (D01650, D0160, or D0180). Within 3 months, scaling and root planing (SRP) is another necessary procedure that may need to be completed. SRP smooths the surface of the root so the gums can reattach properly. The hygienist cleans the root surfaces to remove plaque and calculus.
Doxycycline hyclate, a product available for in-office use, treats gum disease in patients who have 5 mm or deeper pocket depths. Doxycycline hyclate belongs to the class of medicines known as broad spectrum tetracycline antibiotics. It works by killing bacteria or preventing their growth. This product is an antimicrobial agent that is delivered in a slow, localized manner and placed subgingivally in the periodontal pockets to suppress the growth of anaerobic bacteria.14 It is an antibiotic that is used as an adjunct to treat active sites of bacterial growth after SRP. The agent is also used in isolated, recurrent sites.
Fluoride is a common in-office and home product used case by case, determined by the risk level of a patient. The frequency of a patient receiving fluoride treatments should be driven by risk factors, not reimbursement. Typically, most adults will get coverage for one fluoride treatment per year, but they may actually need more frequent treatment. All patients will fall under a risk level ranked low, moderate, or high. Determination of the risk level is based on the patient's age, decay noted in the last 3 years, and factors such as oral hygiene, nutrition, and understanding of the disease process.15 Fluoride is used in the dental office and can also be included in a patient's home care routine. There are many different types of fluoride for patient home care use.16 Home fluoride to help prevent decay and improve periodontal health can be introduced into a patient's daily oral hygiene routine, allowing the patient to maintain a fluoride regimen at home. A prescription strength of 1.1% sodium fluoride, 5,000 ppm, and 5% potassium nitrate can be prescribed by a dentist.15 Another type of fluoride, stannous fluoride, has antimicrobial properties that can be beneficial to patients with periodontal disease. These patients often have recession and related root sensitivity that can be lessened by using stannous fluoride.
A variety of toothbrushes are available for patient use. A toothbrush should be thought of as a disease-fighting home care instrument. Dental plaque is the primary cause of gingivitis and can lead to periodontitis, which is a more serious form of gum disease. Therefore, patients need a tool for removing plaque that is easy to use so the patient is compliant. Home care recommendations are a vital part of a patient's oral health success; selecting the correct home care instrument is equally important. A properly used powered toothbrush can be as effective as manual brushing and flossing.
Another home care tool is a water flosser. There are several different flossers available, including one that is used at the patient's sink and another that is portable and works well in the shower. Flossing is an important part of a daily routine. Patients must floss in order to get to places between their teeth and in the spaces between the teeth and gums that a toothbrush cannot reach. Some patients find that they cannot do an adequate job with floss only. Many patients add a device called a water flosser to their daily routine to improve bleeding and remove food that continues to become trapped between the teeth. Also, a water flosser is useful for when food particles get stuck more frequently in the teeth of patients with braces. Although water flossers may give a patient an extra benefit, regular floss works well, so patients should use the water flosser with regular floss. Interdental brushes are another home care tool that will improve the home care routine. Interdental brushes are safe to use around all types of dental restorations, including implants. They are the only cleaner that can reach very bare root surfaces that often have depressions.
Other tools necessary for home care maintenance include interdental aids, which involve integrating dental flossing, water flossing, and interdental brushes into the patient's home care routine. Patients can also be encouraged to use disclosing tablets at home to display the effectiveness of their toothbrushing technique. Additionally, different types of toothpaste can be recommended for the patient, depending on their situation: for example, toothpastes containing fluoride for preventive treatment, toothpastes for patients with sensitive teeth, and toothpastes with whitening and fluoride. These recommendations should be part of the patient's oral home care plan. Hands-on oral hygiene instructions for each patient will encourage proper techniques and allow the patient to be involved.
A major home care treatment product used for periodontal disease is the periodontal prescription custom tray (Figure 3). Periodontal trays are home care devices for patients with gum disease that assist patients in controlling their periodontal health. The systems allow patients to keep periodontal disease under control between dental visits; the trays are coupled with in-office treatments such as SRP and irrigation. Periodontal trays combined with 1.7% hydrogen peroxide can help patients manage their gingivitis or periodontitis. The customized trays are designed to push a hydrogen cleansing gel deep into each periodontal pocket. The system allows the trays to introduce a localized subgingival delivery of 1.7% hydrogen peroxide and is routinely employed as adjunctive therapy.17 The oral cleansing gel reduces bacteria with each 15- to 20-minute application, while freshening breath and offering a whiter smile.
Hydrogen peroxide has long been used in the treatment of periodontal disease. The first use of hydrogen peroxide in dentistry was in 1913, when it was used to decrease plaque formation and control pyorrhea, or gum disease. The now-known mechanism of antimicrobial action is the release of oxygen, and pathogenic effects are seen in Gram-positive as well as Gram-negative organisms.17 Another mechanism of antimicrobial action is the effect the hydrogen peroxide has on the debridement of bacterial cell walls. A 10-minute exposure to a 1.7% hydrogen peroxide gel penetrates the biofilm slime matrix and debrides the cell walls of in vitro Streptococcus mutans biofilms. Microbiologists have also hypothesized that peroxide delivered and maintained in the sulcus or periodontal pocket releases oxygen and changes the subgingival micro-environment, making it harder for anaerobic bacteria to survive.18 Periodontal prescription trays together with hydrogen peroxide actively fight bacteria buildup inside the periodontal pockets and prevent bacteria from spreading daily. The trays are a critical extension of what is provided in the dental office. They are an effective, non-invasive solution for prevention and treatment of periodontal disease and gingivitis for all patients.
The next part of this article presents three cases in which patients with periodontal disease were treated in the author's office.
Case 1
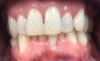
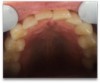
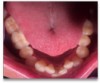
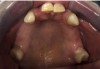
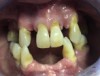
A 68-year-old man presented with a detailed and documented health history revealing severe systemic disease and a previous diagnosis of moderate periodontitis (Figure 4 through Figure 7). He had type 2 diabetes and was receiving nitroglycerin 0.2 mg, sitagliptin 100 mg, cyanocobalamin 1,000 mg, duloxetine 60 mg, tizanidine 2 mg, oxybutynin XL 5 mg, omeprazole 40 mg, and amlodipine 10 mg. At the initial appointment, the patient had a complete set of x-rays and intraoral photographs taken. At the second appointment, periodontal probing was performed, a diagnosis of moderate periodontitis was confirmed, and a treatment plan was discussed and reviewed with patient. The patient had several teeth with probing depths of 5 and 6 mm. SRP was completed on the upper right and left quadrants at the third appointment. Based on a re-evaluation 3 weeks after treatment, doxycycline hyclate 10% was placed in the areas of tooth Nos. 2, 3, 8, and 14 because they still exhibited pockets of 5 mm or greater. The patient was placed on 4-month recall intervals. The patient also was provided with customized periodontal prescription trays with 1.7% hydrogen peroxide. He was instructed to use the prescription customized trays twice daily for 20 minutes each time. The patient was also given a home care program to maintain his periodontal health. He was referred to a periodontist for evaluation. The patient had a consultation with the periodontist but declined to return for treatment. Overall, the patient received the following:
• Periodontal charting (probing)
• Diagnosing
• Treatment planning
• Root planing
• Re-evaluation
• Doxycycline hyclate 10%
Case 2
A 51-year-old man presented for a new patient dental appointment (Figure 8 through Figure 11). His health history revealed mild systemic disease. The patient was receiving simvastatin 20 mg and lisinopril 10 mg. He had a full-mouth set of x-rays and oral photographs taken at his initial appointment. At the next appointment, a debridement was performed. At the third appointment, pocket depths were measured and a diagnosis was made of moderate periodontal disease; a treatment plan was formulated, followed by four quadrants of SRP treatment. The patient was placed on a 3- to 4-month recall cleaning visit. At the fifth appointment, the patient's oral cavity was re-evaluated and doxycycline hyclate 10% was delivered subgingivally in the areas of tooth Nos. 5, 14, 15, 18, and 19. The patient was provided oral hygiene instructions and a home care plan. Several products and methods were introduced, including periodontal prescription trays with 1.7% of hydrogen peroxide placed in the trays and used twice daily for 20 minutes each time. The patient was also placed on stannous fluoride.
Case 3
A 61-year-old woman presented at the office for an initial appointment and examination (Figure 12 through Figure 15). The patient had a previous diagnosis of moderate to advanced periodontal disease. She had periodontal surgery 5 years previously. Her health history revealed mild systemic disease. The patient was receiving omeprazole 40 mg and escitalopram 10 mg. During the visit, full-mouth x-rays (FMX) and oral photographs were completed. At the following appointment, a debridement was completed. The patient returned 3 weeks later for evaluation, periodontal charting, and a diagnosis of moderate to advanced periodontal disease; a treatment plan was developed. At the next two appointments, SRP was completed on both the upper right and left quadrants. Based on re-evaluation 3 weeks after treatment, doxycycline hyclate was placed in the areas of tooth Nos. 9, 11, 19, 20, 28, 29, 30, and 31. The patient was placed on a 3-month recall. She was also prescribed periodontal trays with 1.7% hydrogen peroxide to be used for 20 minutes twice a day. She was also given a master plan for home care maintenance and oral hygiene instructions, including using a powered toothbrush three times daily.
Combining In-Office and Home Care
Each periodontal patient seen in the author's office is treated with the following plan of action, according to their needs:
• FMX
• Oral photographs
• Full-mouth debridement
• Periodontal charting (probing)
• Diagnosing
• Treatment plan
• Root planing
• Re-evaluation
• Doxycycline hyclate 10%
• Periodontal prescription trays
• 3- to 4-month recall schedule and home care program for maintenance between office visits
• Home care maintenance
The most important aspect of a periodontal treatment is getting the patient to accept it. Developing an oral healthcare plan for each patient will increase case acceptance rates and is the key to expanding a hygiene program. It will enable the findings of the assessment to be documented along with any barriers that interfere with oral hygiene home care maintenance, physical or behavioral. Obstacles may include mobility issues, dementia, lack of awareness, and resistance to oral care.19 The home care recommendations are a vital part of a patient's oral health success.
It is important to involve the patient as much as possible in a customized home care plan. This engagement will encourage a patient's involvement and compliance with their oral healthcare. The plan should also include which tools and products are to be used to maintain good oral home healthcare. It is crucial, therefore, for the patient to understand the level of participation that is required on their part to ensure treatment success. The patient also should be aware of the consequences and be educated on the importance of a home care plan to gain control of their periodontal health.
Dental health can be managed properly when recall visits are followed and home care instructions have been given to patients, with an understanding of the goals between each visit. Longer duration between visits results in more dental need and expense. Success in treating, preventing, and maintaining periodontal disease requires in-office treatments and monitoring to establish a relationship with the patient and increase the patient's compliance with their home care. An obstacle that can interfere with home care maintenance is lack of communication with patients.
Communication is an important tool for healthcare providers to use with patients. Dentists and hygienists should convey to patients what is going to happen during the appointment and then, as the appointment proceeds, should discuss with patients what they are doing as it is being done. Finally, findings are discussed; this brings the patient into the diagnosis process so the patient feels a part of the experience. This communication with patients is referred to as co-diagnosing. Communication is the cornerstone of healthcare. Effective communication is not only critical to meeting patient needs but also to providing safe, high-quality, and patient-centered care.20
Conclusion
The success of long-term periodontal health maintenance and treatment is related to the patient's compliance with a home care protocol and timely dental visits. Educating the patient on periodontal disease is a large part of home maintenance. When patients can understand why something is being done and what can be done to prevent further damage, they will be more likely to accept treatment. Providing methods and products to encourage implementation of a detailed home care maintenance program and communicating with and involving the patient in the process and application will provide a successful path to long-term periodontal health.
About the Author
Suzanne R. Mericle, DMD, PC, FDOCS, DASBA, FAAFE, QDAADSM
Private Practice
Saint Simons Island, Georgia
References
1. Benjamin RM. Oral health: the silent epidemic. Public Health Rep. 2010;125(2):158-159.
2. Lazar V, Ditu LM, Curutiu C, et al. Impact of dental plaque biofilms in periodontal disease: management and future therapy. In: Arjunan P. Periodontitis: A Useful Reference. IntechOpen. Published November 15, 2017.
3. Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588.e6.
4. National Institute of Dental and Craniofacial Research. Gum disease. Medline Plus. http://www.nlm.nih.gov/medlineplus/gumdisease.html. Updated May 6, 2020. Accessed May 19, 2020.
5. Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260-267.
6. Bascones-Martínez A, Muñoz-Corcuera M, Noronha S, et al. Host defence mechanisms against bacterial aggression in periodontal disease: basic mechanisms. Med Oral Patol Oral Cir Bucal. 2009;14(12):e680-e685.
7. Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontol 2000. 2010;53(1):12-27.
8. Zhu J, Quyyumi AA, Norman JE, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85(2):140-146.
9. Kinane DF, Lowe GDO. How periodontal disease may contribute to cardiovascular disease. Periodontol 2000. 2000;23(1):121-126.
10. Arboleda S, Vargas M, Losada S, Pinto A. Review of obesity and periodontitis: an epidemiological view. Br Dent J. 2019;227(3):235-239.
11. Neiva RF, Steigenga J, Al-Shammari KF, Wang H. Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol. 2003;30(7):579-589.
12. Overweight and obesity increases risk for periodontitis. News Medical. http://www.news-medical.net/news/20160624/Overweight-and-obesity-increase-risk-for-periodontitis.aspx. Published June 24, 2016. Accessed May 19, 2020.
13. Suvan J, D'Aiuto F, Moles DR, et al. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev. 2011;12(5):e381-e404.
14. Oberoi SS, Mohanty V, Mahajan A, Oberoi A. Evaluating awareness regarding oral hygiene practices and exploring gender differences among patients attending for oral prophylaxis. J Indian Soc Periodontol. 2014;18(3):369-374.
15. Piacentini M, Borghetti RL, de Figueiredo MAZ, et al. Doxycycline: an option in the treatment of ulcerated oral lesions? J Clin Pharm Ther. 2019;44(6):838-843.
16. Norris T. What are the benefits, side effects, and recommendations for fluoride treatment? Healthline. https://www.healthline.com/health/dental-and-oral-health/fluoride-treatment. Reviewed March 13, 2018. Accessed May 19, 2020.
17. Marshall MV, Cancro LP, Fischman SL. Hydrogen peroxide: a review of its uses in dentistry. J Periodontol. 1995;66(9):786-796.
18. Schaudinn C, Gorur A, Keller D, et al. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc. 2009;140(8):978-986.
19. Millennium Dental Technologies, Inc. The secrets to patient acceptance for periodontal treatment revealed. LANAP website. https://www.lanap.com/2016/10/17/patient-acceptance-revealed/. Published October 17, 2016. Accessed June 29, 2020.
20. King A, Hoppe RB. "Best practice" for patient-centered communication: a narrative review. J Grad Med Educ. 2013;5(3):385-393.