You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Peri-implantitis (PI) and peri-implant mucositis are inflammatory lesions identified clinically by redness and swelling of the soft tissue. Where they differ is that peri-implant mucositis—like gingivitis, with which it often co-exists—is reversible, and PI, which by definition is accompanied by bone loss, is not.1
According to the Consensus report of the Sixth European Workshop on Periodontology, there is evidence that the following indicators are associated with peri-implant disease: poor oral hygiene; history of periodontitis; cigarette smoking; diabetes with poor metabolic control; alcohol consumption; genetic traits; and implant surface.1
In light of this information, practitioners should make every effort to minimize these risk factors to prevent complications that threaten implant survival. In addition to eliminating bacterial plaque, practitioners should avoid placing implants whose surfaces or design are prone to breakdown when exposed to inflammation/bacterial plaque and ensure meticulous cement removal. Examples of these types have included implants with hydroxyapatite and plasma-sprayed titanium (TPS) surfaces. Finally, if prevention fails, practitioners should promptly initiate effective therapy to preserve the dental implant, and if necessary remove it as safely and noninvasively as possible when no other options are available.
Prevention Steps
Plaque Removal
Numerous studies suggest that the first step to preventing problems with implants lies in eliminating plaque/biome. A study by Salvi et al2 found that cessation of oral hygiene leads to gingivitis around teeth and peri-implant mucositis around dental implants in humans, although the inflammatory response to plaque was stronger in the peri-implant tissue compared to its gingival counterpart; furthermore, the inflammation was found to be reversible at the biomarker level as 4 weeks of resumed plaque control did not yield pre-experimental levels of gingival and peri-implant mucosal health, suggesting that longer healing periods are needed.2 Similar findings in dogs were reported in an earlier publication by Pontoriero and others.3
Cement Removal
In the interest of preventing PI, practitioners should make every effort to ensure complete cement removal. Wadhwani4 noted that some types of cements commonly used for implant-supported prostheses have poor radiodensity and may not be detectable following radiographic examination,4 leading to complications with implants, as with tooth-supported restorations. The positive relationship between excess cement and peri-implant disease was also noted in a study by Wilson5 of 39 consecutive patients with implants exhibiting clinical and/or radiograph signs of peri-implant disease. The investigators found evidence of excess cement in 34 of the 42 peri-implant diseased sites, but none among any of the 20 controls. After removal of excess cement, these signs were absent in 74% of the test implants.5 Moreover, this study suggests that not only cement but plaque as well is a cause of peri-implant disease as 8 of the 42 affected implants had no cement.
Recordkeeping
While the Wilson5 study demonstrated that cement removal was not sufficient in all instances to restore the implants back to health, it also suggested that adequate records—a key to prevention—are essential for tracking and allowing early identification. Practitioners should take care to document demonstrable changes in probing depths and the appearance of the soft tissue that can indicate problems. A recent whitepaper by the American Academy of Periodontology suggests that a baseline radiograph should be obtained at the time of implant placement to verify bone levels, then again at restoration to document both bone level changes and where possible if complete cement removal had been achieved.6 Moreover, subsequent radiographs should also be taken in the event of soft tissue attachment level changes or significant tone and color change if the prosthesis prohibits probing.
PI Treatment Approaches
Given the common etiologies of periodontitis and peri-implantitis, the author believes there should be no differences in approaches to treatment of periodontal and peri-implant disease, and that efforts to save an implant should be as vigorous as those to save a tooth—using aggressive treatment, including regenerative surgery—with extraction used after other appropriate efforts have failed. The literature details the use of various approaches for prevention and treatment.
Supportive Periodontal Therapy
That periodontally compromised implant patients can benefit from supportive periodontal therapy (SPT) was clear from the 10-year results of a three-arm prospective cohort study on implant patients, who had a higher rate of implant failure when not completely adhering to supportive periodontal care.7 Their report suggested that such therapy is a key factor in controlling reinfection and limiting biologic complications, thus enhancing long-term outcomes.7
A report by Levy at al8 concluded that “periodontal surgery appears to be an important part of the armamentarium to control periodontal infections.” Their study involved 18 patients with chronic periodontitis who received initial therapy including scaling and root planing followed by surgery (apically positioned flaps) at sites with probing depths > 4 mm. Calling the 5-mm pocket an apparent “reservoir for bacteria to infect the other teeth,” they determined that “reduction in pocket depth by surgical means and the associated decrease in reservoirs of periodontal pathogens may be important in achieving sustained periodontal stability.”8 A study by Matuliene and colleagues in 2008 reached similar conclusions, noting that when residual probing depths are 6 mm or more, reservoirs of bacteria that can infect other sites remain.9
However, the limitations of SPT are apparent in studies whose conclusions identify patients least likely to benefit. Pjetursson et al10 followed up 70 patients who received comprehensive periodontal treatment after implant therapy for a mean period of 7.9 years. Using a threshold level of 5 mm probing pocket depth and bleeding on probing, 22.2% of the patients and 38.6% of the implants were deemed affected by disease. The study concluded that in periodontitis-susceptible patients, residual pockets greater than 5 mm at the end of active periodontal therapy represent a significant risk for the development of peri-implantitis and implant loss. They also concluded that patients experiencing reinfections are at greater risk for PI and implant loss than periodontally stable patients.10
A 245-case retrospective study in Sweden that used a 7-mm PPD threshold plus pus and bleeding to define PI reported that in 54.7% of the patients, it was not feasible to arrest progression of PI.11 The study also found that significantly related to implant failure were smoking and smoking dose and early disease development. These authors concluded that peri-implant health may not be easy to re-establish, especially in patients who develop disease early, and recommended that homogenous treatment protocols—rather than empirical treatment attempts—should be adopted.11
Mechanical Nonsurgical Treatment
Mechanical nonsurgical treatment alone has been found to be inadequate for eliminating the causitive agents of periodontitis from the root surface12 and in the case of peri-implantitis, had no benefit for reducing probing pocket depth.13 The study by Stambaugh and others evaluated seven human teeth with 42 pockets treated with subgingival scaling by clinically excellent dental professionals.12 They reported that it was not possible to achieve in one instrument session a root surface free of those agents responsible for most periodontal disease when the pocket depth is greater than 4 mm.12
Renvert and his colleagues13 studied mechanical nonsurgical treatment of peri-implantitis. Their double-blind randomized longitudinal clinical study of 37 subjects treated these individuals with either a titanium hand-instrument or with an ultrasonic device and found no group differences in the treatment outcomes among the 31 who completed the study. While plaque and bleeding scores improved, no effects on pocket were identified. Both treatments failed to change the bacterial load.13
Surgical Approaches
Tissue Grafting
More aggressive approaches to the correction of soft tissue deformities—where keratinized tissue is absent leading to the establishment of plaque—in peri-implant mucositis and peri-implantitis sometimes involves soft tissue grafting, as described by Roos-Jansåker, who demonstrated the importance of responding promptly to peri-implantitis to prevent bone loss progression.14
Combined Surgical and Antimicrobial Treatment
A study by Leonhardt et al15 that evaluated combined surgical and targeted antimicrobial treatment for peri-implantitis lesions in humans examined 26 implants clinically, microbiologically, and radiograpically at 6 months, 1 year, and 5 years. Among the treated implants, despite this therapy and retreatment, this targeted antimicrobial strategy was found to be only 58% successful.15
Regeneration
Rather than removal of an implant with moderate–advanced PI16 or a tooth with moderate–advanced periodontitis, the author has successfully used the same solution—a properly performed regenerative approach to bone loss—to achieve improvement/eradication of both.17,18 Regeneration has been demonstrated in humans when using a variety of techniques to treat periodontitis,19 and it may also be used on a diseased implant surface, according to a systematic review by Renvert et al.20 Seeking existing evidence of re-osseointegration after treatment of peri-implantitis at contaminated implant surfaces, the authors identified a total of 25 animal studies that fulfilled the inclusion criteria.20 Based on these studies, they determined that, with therapy, it is possible to achieve re-osseointegration on a previously contaminated implant surface with experimentally induced peri-implantitis defects, but that the amount varied considerably from one study to the next. While the authors concluded that no method could predictably achieve resolution of the peri-implant defect, they maintained that implant surface characteristics may influence the degree of re-osseointegration and that surface decontamination alone cannot achieve substantial re-osseointegration on a previously contaminated implant surface.20
Regeneration Technique
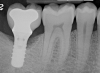
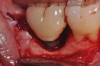
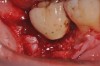
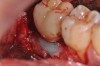
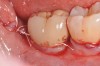
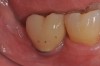
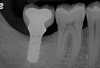
A report by father–son colleagues Stuart and Scott Froum, along with the author,18 described a promising regenerative approach to the successful management of peri-implantitis, with an emphasis on surface decontamination (Figure 1 through Figure 7). The “seven essential factors” identified included:
1. Flap access to ensure adequate blood supply
2. Surface decontamination
3. Defect debridement, using a biologic agent on implant surface
4. Defect fill, using freeze-dried bone allograft (FDBA) and/or anorganic bovine bone
5. Coverage, using absorbable membrane or a subepithelial connective tissue graft
6. Coronal positioning of flaps, providing complete coverage of membrane/graft
7. Professional maintenance and excellent homecare
Surface Decontamination in the Literature
Decontamination of the implant surface is essential to the success of regenerative procedures. The findings cited below are based on studies of air powder abrasive and citric acid, plus combinations of these agents with the addition of sterile saline.
Tastepe et al21 conducted a systematic review of the literature on air powder abrasive treatment as an implant surface cleaning method. The 27 studies that met the inclusion criteria included 19 in vitro studies, three in animals, three in human case series, and one a randomized control trial reported in two articles. The review reported that air powdered abrasive for decontamination of an implant surface is an important component to regenerative treatment for PI treatment.
A systematic review of the literature by Ntrouka et al22 examined the effect of chemotherapeutic agents on biofilm-contaminated titanium surfaces. Among 2425 unique papers identified, only four publications met all eligibility criteria. Despite the scarcity of “robust data,” the authors “cautiously concluded” that citric acid is the chemotherapeutic agent with the highest potential for the removal of biofilms from contaminated titanium surfaces in vitro, although it does not achieve complete removal.22
Further support for citric acid has most recently been provided by Gamal et al,23 who evaluated micro- and nano-hydroxyapatite (NHA) blended clot adhesion to citric acid-conditioned peri-implantitis–affected surfaces in vitro. These authors compared the citric acid–etched surfaces to non-etched implant surfaces from 40 hopeless implants designated for removal from patients with peri-implantitis. Based on scanning electron microscopy, the authors concluded that citric acid conditioning improves NHA-blended clot adhesion to peri-implantitis affected implant surfaces.
Combined Approaches
Given that intra-bony defects around both teeth and implants come in all shapes and sizes, treatment might require a combination of approaches.
As shown in a study by Schwarz et al, implantoplasty can be part of PI therapy.24 The objective of their 10-patient (n=13 implants) study was to evaluate the clinical outcomes following a combined surgical therapy for advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. In the study, patients underwent access flap surgery that included implantoplasty at supracrestally exposed implant portions, and augmentation of the intrabony components using a natural bone mineral and a native collagen membrane after surface decontamination. A subepithelial connective tissue graft was harvested from the palate and adapted to the wound area to support transmucosal healing. Clinical parameters were recorded at baseline and after 6 months. The authors concluded that “the combined surgical procedure investigated may be effective in controlling advanced PI lesions without compromising the overall esthetic outcome in the short term.”24
Failed Implant Removal
Despite the best efforts of the dental team, sometimes there is no choice other than to remove the failed implant. When this is the case, the goals of removal are to remove the implant safely and to minimize the loss of bone during the implant removal process.
There are a number of different techniques for removing a failed integrated implant. Froum and colleagues25 describe an algorithm clinicians can use to determine the most appropriate minimally invasive method—taking into account clinical factors such as anatomical conditions, implant design, condition of implant connection, bone quality, and remaining amount of bone integrated to the implant body. The methods listed in order of least to most invasive are: torque wrench adaptor, piezoelectric instrument, scaling tip, forceps, high speed burs, and trephine burs.25
Preparing for Success
The bottom line is that preventing the biologic complication of peri-implantitis while providing for predictable long-term success for dental implants is predicated on the absence of periodontal disease, performance of effective daily oral hygiene on the part of the patient, and consistent attendance to prescribed maintenance. This was demonstrated in an ahead-of-print article by Lagervall and Jansson.26 This retrospective longitudinal study on a referral population involved 150 patients and 382 implants with peri-implantitis, defined as PPDs >5 mm or bone loss comprising at least three threads of the implant. Periodontal flap surgery with osteoplasty was the most common type of therapy (47%); regenerative surgery procedures with bone substitute materials were implemented in 20% of the cases. Treatment was successful in nearly 70% of implants; the success rate was significantly lower for those diagnosed with severe periodontitis, severe marginal bone loss around the implants, poor oral hygiene, and low compliance.26
Summary
In summary, regenerative therapy remains a predictable method by which teeth with periodontitis and implants with peri-implantitis can be maintained in health, comfort, and function with appropriate esthetics. Approaches to regenerative care for both teeth and implants are the same. The complexity of the lesion’s morphology on a tooth or implant may dictate taking a more layered approach which includes regenerative therapy rather than giving up altogether on their being maintained. However, there is no substitute for the experience and technical excellence offered by specialists who provide this type of therapy expertly and routinely in their practices.
REFERENCES
1. Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(suppl 8):282-285.
2. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
3. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-259.
4. Wadhwani C, Hess T, Faber T, et al. A descriptive study of the radiographic density of implant restorative cements. J Prosthet Dent. 2010;103(5):295-302.
5. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
6. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84(4):436-443.
7. Roccuzzo M, De Angelis N, Bonino L, Aglietta M. Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: implant loss and radiographic bone loss. Clin Oral Implants Res. 2010;21(5):490-496.
8. Levy RM, Giannobile WV, Feres M, et al. The effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota: 12-month data. Int J Periodontics Restorative Dent. 2002;22(3):209-219.
9. Matuliene G, Pjetursson BE, Salvi GE, et al. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol. 2008;35(8):685-695.
10. Pjetursson BE, Helbling C, Weber HP, et al. Peri-implantitis susceptibility as it relates to periodontal therapy and supportive care. Clin Oral Implants Res. 2012;23(7):888-894.
11. Charalampakis G, Rabe P, Leonhardt A, Dahlén G. A follow-up study of peri-implantitis cases after treatment. J Clin Periodontol. 2011;38(9):864-871.
12. Stambaugh RV, Dragoo M, Smith DM, Carasali L. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1(5):30-41.
13. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36(7):604-609.
14. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33(4):296-301.
15. Leonhardt A, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol. 2003;74(10):1415-1422.
16. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
17. Rosen PS, Toscano N, Holzclaw D, Reynolds MA. A retrospective consecutive case series using mineralized allograft combined with recombinant human platelet-derived growth factor BB to treat moderate to severe osseous lesions. Int J Periodontics Restorative Dent. 2011;31(4):335–342.
18. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year followup. Int J Periodonics Restorative Dent. 2012;32(1):11-20.
19. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8(1):227-265.
20. Renvert S, Polyzois I, Maguire R. Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res. 2009;20(suppl 4):216-227.
21. Tastepe CS, van Waas R, Liu Y, Wismeijer D. Air powder abrasive treatment as an implant surface cleaning method: a literature review. Int J Oral Maxillofac Implants. 2012;27(6):1461-1473.
22. Ntrouka VI, Slot DE, Louropoulou A, Van der Weijden F. The effect of chemotherapeutic agents on contaminated titanium surfaces: a systematic review. Clin Oral Implants Res. 2011;22(7):681-690.
23. Gamal AY, Abdel-Ghaffar KA, Iacono VJ. A novel approach for enhanced nanoparticle-sized bone substitute adhesion to chemically treated peri-implantitis-affected implant surfaces: an in vitro proof-of-principle study. J Periodontol. 2013;84(2):239-247.
24. Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series [published online ahead of print January 27, 2013]. Clin Oral Implants Res. doi: 10.1111/clr.12103.
25. Froum S, Yamanaka T, Cho SC, et al. Techniques to remove a failed integrated implant. Compend Contin Educ Dent. 2011;32(7):22-26,28-30.
26. Lagervall M, Jansson LE. Treatment outcome in patients with peri-implantitis in a periodontal clinic- a retrospective study [published online ahead of print December 13, 2012]. J Periodontol. doi:10.1902/jop.2012.120555.
ABOUT THE AUTHOR
Paul S. Rosen, DMD, MS
Clinical Associate Professor of Periodontics,
University of Maryland Dental School,
Baltimore, Maryland;
Private Practice,
Yardley, Pennsylvania
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com