You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The options for cementing ceramic restorations in indirect dentistry have steadily expanded. To achieve optimal results, clinicians need to understand the different categories of cements, their strengths and weaknesses, and their degree of compatibility with the chosen ceramic. Even more paramount is knowing which type of cement to use and how to properly use it based on the level of retention provided by the tooth preparation form.
The first consideration for long-term success in indirect dentistry is to determine whether the preparation is retentive or non-retentive. If the preparation is retentive (ie, the ferrule is adequate, the preparation walls are ≥ 2 mm tall,1 and the proper resistance-retention form is present), all cement types can be used predictably and with success. When the preparation is providing most of the retention, the focus is placed on the luting agent. Ceramic primers can still be used, but are not essential to the success of the restoration. However, if a resin cement is the luting agent of choice, the proper ceramic primer should always be incorporated into the ceramic preparation prior to cementation.
Non-retentive preparations pose more of a challenge. When ferrule is inadequate, preparation walls are not as high, and the resistance-retention form is less than optimal, the bond of the cement to both the tooth and the ceramic becomes more critical. Factors that must be considered include how both the restoration and the tooth will be prepared for bonding and what type of cement is indicated. Bonding is also essential for retentive preparations when a ceramic crown is less than 1.5 mm in thickness.
Ceramic Cementation for Retentive Preparations
For retentive preparations, three basic types of cements provide predictable options: self-adhesive resin cements, resin-modified glass-ionomer (RMGI) cements, and bioceramic cements.
Self-Adhesive Resin Cements
Bond strengths for these types of resin cements are usually relatively low (ie, approximately 10 MPa to 20 MPa), which is inadequate for non-retentive preparations.2 However, they can be used when proper ferrule and resistance-retention form are present.
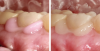
In these cases, the tooth preparation provides the main source of retention rather than the bond between the cement and the tooth/ceramic. Self-adhesive resin cements are appropriate when some bond is desired but the preparation is providing the primary retention. This includes situations in areas of esthetic concern for which other luting agents would be too opaque and certain translucent or colored cements are desired. There are still issues regarding isolation, and the tack-cure hardness of some self-adhesive resin cements has also come into question. For this reason, products are being introduced with a longer gel phase and a wider tack-cure window. Some self-adhesive resin cements even offer visual cues, such as changing color from pink to white as polymerization occurs (Figure 1). But even with these improvements, self-adhesive resin cements should only be used when the tooth preparation is retentive with proper ferrule.
RMGI Cements
RMGI cements are easy to use and have a long history of offering predictable results. They release fluoride, and because they set in the presence of moisture, they can provide a good marginal seal in areas that are difficult to isolate. Conversely, if too much moisture is present, RMGI cements can uptake that water, resulting in a less-than-optimal set that can lead to premature breakdown and failure. RMGI cements are acidic in nature and may expand over time. Although perfectly adequate for most retentive preparations regardless of the ceramic being cemented (eg, zirconia, lithium disilicate, porcelain), RMGI cements are not indicated for non-retentive tooth preparations due to low bond strengths that are comparable to those of self-adhesive resin cements.
Bioceramic Cements
All luted restorations have some degree of cement space, no matter how microscopic, that remains between the ceramic and the tooth. Although this has been an accepted part of indirect restorative dentistry, a new category of cements with the potential to seal these gaps has emerged that stems from the success of mineral trioxide aggregate (MTA)-type materials. These bioceramic cements have calcium aluminate, which is similar to calcium silicate—the main component in MTA materials. Like MTA materials, these bioceramic cements have characteristics that allow new hydroxyapatite layers to form between the ceramic and the tooth structure. With a neutral/basic pH of 8.5 and large amounts of calcium, bioceramic cements will not break down over time in the acidic oral environment. Because of their unique chemistry and calcium ions, bioceramic cements form apatite crystals in the presence of phosphates, including the phospholipids found in saliva, the phosphates in the tooth structure, and those on the ceramic itself.3
Unfortunately, shear bond strengths of bioceramics are not extremely high. On enamel, the shear bond strength of a leading bioceramic cement was found to be similar to that of an RMGI cement.4 Although its bond with dentin is a bit stronger than an RMGI cement, bioceramic cement is not comparable in strength to resin cement and thus, is not recommended for non-retentive preparations. When indicated, bioceramic cement does bond well to zirconia though, largely because of the ceramic's affinity with phosphate groups.
Recently, clinicians have begun questioning whether or not bioactive dental cements are able to seal marginal gaps. The results of a preliminary investigation in 2015 were encouraging.5 The researchers found no evidence of marginal gap occlusion for three conventional cements that were evaluated under simulated aqueous physiologic conditions. However, the two bioactive surface apatite-forming cements that were studied did appear to seal or reseal the artificial marginal gaps (Figure 2), suggesting a new functional property for bioactive dental materials. The researchers noted that the ability to significantly and predictably improve marginal stability could enhance the survival and serviceability of dental restorations. Figures 3 through 8 illustrate the use of a bioceramic cement to secure a single maxillary lithium disilicate crown.
Ceramic Cementation for Non-Retentive Tooth Preparations
When a tooth preparation lacks proper ferrule and resistance-retention form, several steps are required for the predictable cementation of ceramics. As indicated previously, the clinician must consider the bonding surface of both the ceramic and the tooth as well as the type of cement to be used.
When priming a ceramic restoration, the first consideration should be the substrate type. Two main categories exist: glass-based ceramics (eg, lithium disilicate, feldspathic porcelain, leucite reinforced) and zirconia/metal-based ceramics (eg, zirconia, alumina, noble metals). Both types require the use of a primer, but each has different needs. Glass-based ceramics are etchable by hydrofluoric acid, whereas zirconia/metal-based restorations are not. Silane can be used for glass-based ceramics, but a zirconia/metal-specific primer must be used for zirconia/metal-based restorations.
Priming Glass-Based Ceramics for Cementation on Non-Retentive Preparations
When priming glass ceramics, such as lithium disilicate, the use of hydrofluoric acid etching in combination with a silane primer creates a durable bond and significantly increases the bond strength when compared with other methods. A study conducted in 2005 found that postthermocycling bond strength varied from 0 MPa to 61 MPa for various surface preparations of a lithium disilicate-based ceramic material and that the combined application of hydrofluoric acid and proprietary silane primer provided the best results.6 Essentially, the primer plays a role similar to that of a bonding agent on teeth. The glass-based ceramic is hydrophilic, whereas the resin cement is hydrophobic. The primer creates a hybrid layer between the two that enables the cement to spread and coat the surfaces very evenly. The silane primer acts both micromechanically and chemically. The micromechanical action occurs because the hydrofluoric acid etching creates voids within the glass-based ceramic and the silane fills those voids, similar to bonded resin tags within dentinal tubules that have been etched and opened. The chemical action occurs because application of the hydrofluoric acid releases a number of different ions to which the phosphate groups in the silane then bond. Chemically, the silane bonds not only to the ceramic, but also to the cement.
In the process of preparing a glass-based ceramic restoration for cementation, the clinician may wish to begin by sandblasting the surface to be cemented. Although manufacturers of lithium disilicate material discourage sandblasting, citing concerns about potential fractures, many clinicians have been sandblasting lithium disilicate crowns for years without experiencing any problems. Acid etching should not exceed 30 seconds. Although early porcelains could be acid-etched for up to 2 minutes, etching lithium disilicate crowns for more than 30 seconds has been found to decrease the bond strength.7 Inadvertently etching lithium disilicate restorations for longer than 30 seconds can result in an overly frosty appearance. Removal of the excess particles created by over-etching can be achieved by placing the crown in an ultrasonic bath.
Following a try-in, any necessary adjustments can be made. Once adjusted, the crown is ready to be cleaned with standard phosphoric acid etching. After 30 seconds of cleaning, several coats of silane primer should be applied. The primer should be allowed to air-dry for 30 seconds, or a blow dryer can be used to provide clean, warm oil-free air. When the restoration is dry, the resin cement can be applied.
Another approach is to have the laboratory do the sandblasting and acid etching; however, good communication with the lab about how the crown was prepared is essential. One final consideration is that some manufacturers state that their bonding agent can double as silane.
In fact, this is only recommended if the bonding agent contains 10-methacryloyloxydecyl dihydrogen phosphate (MDP). Otherwise, clinicians should consider using a porcelain silane that is specifically manufactured for glass-based ceramics. Typically, it comes in both single- and double-bottle formulations. If the practice is priming a high volume of restorations, a single bottle formulation should suffice; however, a two-bottle system in which the primer is mixed prior to use is a better choice if the product may be sitting on the shelf for some time.
Priming Metal-Based Ceramics for Cementation on Non-Retentive Preparations
Priming zirconia- and metal-based ceramics differs from that of glass-based ceramics because it is not possible to micromechanically etch zirconia. Instead, the bonding action of zirconia is strictly chemical. The polycrystalline structures of zirconia- and metal-based ceramics are impervious to hydrofluoric acid etching. Although some manufacturers recommend sandblasting zirconia-based ceramics, research has found this approach to be ineffective at changing zirconia's morphologic microstructure.8 Nonetheless, the use of appropriate primers can improve the bond strength of such ceramics. The use of a phosphonic acid monomer or a phosphate ester monomer has been found to improve the bonding of resin to zirconia ceramic.9 In 2011, another study confirmed that silane treatment had no positive impact on the bond strength of polished zirconia. But after silicatization with a blast-coating agent (CoJet™ Sand, 3M ESPE), zirconia's bond strength was slightly increased, and the use of phosphate/carboxylate-based primer doubled the bond strength.10
Zirconia has a high affinity for phosphate groups; consequently, the phospholipids in saliva can easily contaminate zirconia surfaces. When zirconia was first introduced, manufacturers were recommending cementation with certain self-adhesive resin cements, but the crowns tended to rapidly debond. Although doctors would quickly try them in, clean them off, and then apply self-adhesive resin cement, the crowns were coated by the phospholipids in saliva, which occupied all of the phosphate spots needed for the resin cement and led to debonding. To avoid this, it is important to use a cleaning paste product rather than a phosphoric acid etching one that will further contaminate the surface. A concentrated form of liquid zirconia can be used to remove the phospholipids from the zirconia surface; 20 seconds is the recommended cleaning time (Figure 9). After a zirconia primer is applied, it should be allowed to set for 30 seconds before the final step of resin cement application. As with glass-based ceramics, the primer develops a chemical bond to both the zirconia and the cement. Light-cured resins and universal resins are both acceptable cement choices, as long as an accompanying adhesive is being used.
Bonding the Surface of the Tooth
When preparing a tooth to receive a bonded ceramic restoration, a proper bonding protocol must be followed. If a large amount of enamel is present, an etch-and-rinse technique with phosphoric acid followed by an application of primer/adhesive is the preferred method. These situations include those involving inlays, onlays, and veneers. In most bonding situations where a full-coverage restoration is indicated, self-etching adhesive systems have been shown to work extremely well (Figure 10). This is because the entire preparation is now in dentin, where self-etching adhesives have the strongest microtensile bond strengths.11,12
In a bonding protocol, while preparing the tooth for the restoration, the appropriateness of using chlorhexidine to adequately cleanse the tooth has been questioned. A study published in 2013 examined the effect of chlorhexidine on the bonding durability of two self-etching adhesives.13 According to the results, chlorhexidine use significantly decreased the initial bond strength of the self-etching adhesives. After aging, however, no significant difference was evident between the bond strengths of the chlorhexidine-treated and control groups. Regardless, the significant decrease in the initial bond strength is sufficient to argue against chlorhexidine's use with a self-etching adhesive. Alternatively, when using a total-etch adhesive, the use of chlorhexidine appears not to have any deleterious effect.
Bonding the Ceramic and the Tooth Together
After cleaning the tooth, but before bonding the tooth surface and the crown, isolation is mandatory to reduce the risk of contamination.14 Once the tooth has been cleaned and isolated and the ceramic has been prepared, the bonding procedure can begin. The choice of a self-adhesive resin, RMGI, bioceramic, or resin cement is now critical.
Although many different types of cements are available, resin cements provide greater predictability for use with non-retentive preparations. Self-adhesive resin cements, RMGIs, and bioceramic cements should be indicated only for retentive preparations due to their lower bond strengths.2 Furthermore, using a cement and adhesive from the same manufacturer is strongly recommended because they are designed to work together, which increases predictability.
After application of the resin cement to the ceramic, the restoration should be seated and tack-cured for just a few seconds (ie, enough to start the polymerization process without the cement becoming too hard) (Figure 11). After flossing, full light-curing can be completed buccally and lingually for 20 seconds each. It is important to have the patient bite down on cotton for the total cure time recommended by the manufacturer (eg, 4 minutes) before any occlusal adjustments are made. This is necessary to allow the chemical component of a dual-cure system to fully polymerize. A cautionary note regarding tack-curing is that it is important to understand the position of the osseous crest relative to the tooth. A low or high osseous crest can increase the risk of cement becoming stuck underneath the contact. Taking a routine radiograph after every cementation can help to ensure that any retained cement is identified and removed.
Conclusion
In summary, clinicians should decide which cement to use for each indirect restorative case based upon the preparation style, not solely on their preferences. For retentive preparations, three main categories of cements are satisfactory: self-adhesive resin cements, RMGI cements, and bioceramic cements. With the potential to create new nanocrystalline formations on the tooth structure and on the ceramic, the bioceramic cement in particular appears to hold great promise for dentistry. For non-retentive preparations, resin cement should be used in conjunction with a primer that is appropriate for the substrate material.
About the Author
Greg Gillespie, DDS
Private Practice
Vancouver, Washington
References
1. Shillingburg HT Jr, Hobo S, Whitsett LD, et al. Fundamentals of Fixed Prosthodontics. 3rd ed. Carol Stream, IL: Quintessence Publishing; 1997:191.
2. Viotti RG, Kasaz A, Pena CE, et al. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. J Prosthet Dent. 2009;102(5):306-312.
3. Pameijer CH. Ceramir™ Crown & Bridge luting agent-a treatise on biocompatibility. August 2009. http://ceramir.se/BinaryLoader.axd?OwnerID=34f41d5a-9511-49fd-908a-2d973293c9b7&OwnerType=0&PropertyName=relatedArticles&FileName=Ceramir+C%26B-+A+treatise+on++biocompatibility-.pdf&Attachment=True. Accessed August 18, 2016.
4. Jefferies SR, Loof J, Pameijer CH, et al. Physical properties of XeraCem™ [abstract]. J Dent Res. 2008;87(spec iss B): Abstract 3100.
5. Jefferies SR, Fuller AE, Boston DW. Preliminary evidence that bioactive cements occlude artificial marginal gaps. J Esthet Restor Dent. 2015;27(3):155-166.
6. Nagai T, Kawamoto Y, Kakehashi Y, Matsumura H. Adhesive bonding of a lithium disilicate ceramic material with resin-based luting agents. J Oral Rehabil. 2005;32(8):598-605.
7. Zogheib LV, Bona AD, Kimpara ET, McCabe JF. Effect of hydrofluoric acid etching duration on the roughness and flexural strength of a lithium disilicate-based glass ceramic. Braz Dent J. 2011;22(1):45-50.
8. Borges GA, Sophr AM, de Goes MF, et al. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J Prosthet Dent. 2003;89(5):479-488.
9. Kitayama S, Nikaido T, Takahashi R, et al. Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent Mater. 2010;26(5):426-432.
10. Chen L, Suh BI, Kim J, Tay FR. Evaluation of silica-coating techniques for zirconia bonding. Am J Dent. 2011;24(2):79-84.
11. van Dijken JW. A prospective 8-year evaluation of a mild two-step self-etching adhesive and a heavily filled two-step etch-and-rinse system in non-carious cervical lesions. Dent Mater. 2010;26(9):940-946.
12. Walter R, Swift EJ Jr, Boushell LW, Braswell K. Enamel and dentin bond strengths of a new self-etch adhesive system. J Esthet Restor Dent. 2011;23(6):390-396.
13. Shafiei F, Alikhani A, Alavi AA. Effect of chlorhexidine on bonding durability of two self-etching adhesives with and without antibacterial agent to dentin. Dent Res J (Isfahan). 2013;10(6):795-801.
14. Powers JM, Finger WJ, Xie J. Bonding of composite resin to contaminated human enamel and dentin. J Prosthodont. 1995;4(1):28-32.