You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
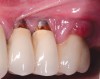
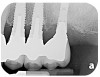
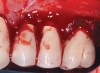
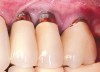
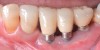
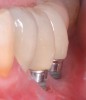
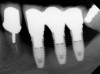
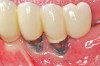
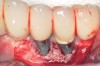
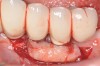
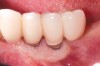
Dental implants have revolutionized the way in which clinicians treat and restore partially and fully edentulous patients and have demonstrated success for managing a broad range of clinical dilemmas. While implants have been highly predictable and have achieved long-term success, they are not immune from complications associated with improper treatment planning, poor surgical and prosthetic execution, material failure, and inadequate or infrequent maintenance. Among these problems are the biologic complications of peri-implant mucositis and peri-implantitis, which are inflammatory conditions in the soft and/or hard tissues surrounding dental implants (Figure 1 through Figure 4).1,2
A recent systematic review, based on a European consensus conference, revealed that the prevalence of peri-implant mucositis and peri-implantitis has ranged from 19% to 65%,3 as no determination of the threshold for diagnosing the disease exists.4 To further facilitate this, a classification scheme was proposed5 with treatment algorithms being based on severity.6 Although findings from a number of articles, including case reports, case series, and controlled clinical trials, attest to the ability to successfully treat these conditions,6-12 it is far better and easier to prevent them from arising. The dental community’s understanding about pathologic peri-implant bone loss continues to evolve, thus requiring further reevaluation.
Historically, treatment of peri-implantitis has lacked predictable success,3,6 prompting the question of whether it is better to treat and retain an implant affected by peri-implantitis or to extract it and start anew, or go in a different direction. Recent articles, most of which are case reports13,14 and case-series cohorts,15 have reported effectiveness in treating these biologic complications with various approaches. However, systematic reviews indicate a lack of evidence to support any one approach.16,17 Other recent reports11,12 have demonstrated outstanding results with a regenerative algorithm, enabling the maintenance of dental implants once considered to be hopeless. While it is encouraging to know that emerging options exist for managing these biologic complications around dental implants, clearly the best approach is to prevent the problem rather than have to face the challenges of treatment.
In 2006, a landmark periodontal workshop held in Europe outlined seven evidence-based factors associated with peri-implantitis, all with varying levels of impact.1 Those risks with strong evidence included poor oral hygiene, a history of periodontitis, and cigarette smoking. Factors with limited information as risk indicators were diabetes mellitus with poor metabolic control and alcohol consumption, while conflicting and limited evidence existed for genetic traits and implant surface. The final recommendations centered on plaque management and limiting those factors that would enhance the development and propagation of peri-implantitis.
More recently, other factors have come to light that offer evidence to merit their inclusion as risk indicators, while some of the original items that were suggested for peri-implantitis should be deleted from the list. Some of these emerging additions may include poor implant positioning; inadequate gingiva; poor fit of the prosthetic components used, ie, micromotion or the use of aftermarket parts; the presence of foreign material such as dental cement or titanium particles; the reuse of healing abutments; and hypertension. These emerging issues are discussed in the following sections.
Poor Implant Positioning
Studies have demonstrated that much like teeth, dental implants have a certain biologic dimension established following their healing after surgery and much of this understanding comes from evidence obtained from animal studies.18,19 However, in a seminal article on implant placement, Salama et al20 further highlighted the concept of bone surrounding an implant. A violation to the dimension of bone present in any of the buccal-palatal, mesial-distal, or apical-coronal directions will lead to bone loss (Figure 5 through Figure 7). This relates to the blood supply necessary to maintain the bone surrounding a dental implant. If an implant is placed in a position in which the minimal bone dimensions are not respected, crestal loss can be anticipated. While this may represent a contained lesion and remain static, it may not always be the case. In implants designed with a surface roughened to the top, suboptimal plaque control can lead to ongoing bone loss, because the exposed roughened surface is challenging to clean and will act as a plaque-retentive feature.21
Poor implant positioning can also compromise the patient’s plaque-control efforts. While the location of the bone may not allow for optimal implant placement, unintended negative consequences can result from the prosthesis superstructure impeding the patient’s attempts at controlling plaque. Typically, the use of pink porcelain or composite to close apical or interproximal spaces caused by restoration of a malposed implant will not allow patient access for adequate homecare.
Insufficient Amount of Gingiva
Long considered inconsequential for maintaining the health of a dental implant, the amount of gingiva surrounding an implant can be a significant factor. The initial belief that gingiva was unimportant or only a consideration in circumstances in which inflammation was present stems from earlier work with dental implants.22 The original intent of dental implants was to rehabilitate patients who had become crippled by oral disease, leaving them with few, if any, options on regaining masticatory function. In these patients, implants were placed into basal bone with little or no gingiva on the remaining ridge. Providing gingiva was impossible, as this would have caused a total “degloving” of the jaw. Moreover, the basal bone was usually broad enough that bone dimension was compromised only in an apical-coronal dimension.
Since those early days of implant dentistry, the situational use of implants has greatly expanded with more being placed into the woven-bone segment of the jaw. Systematic reviews have shown a high association between a lack of “keratinized tissue” and peri-implant soft-tissue inflammation.23,24 This, in part, relates to an absence of supracrestal gingival fibers present in the soft tissue that in health insert into teeth to help withstand inflammatory insult. In the case of an implant, these fibers are parallel to the implant, thus leaving a hemidesmosomal attachment as the only line of defense to seal the outer environment from the underlying soft and hard tissues.
A thinner gingival biotype is also a sign that the underlying bony housing may be thin; this relates to the concept of blood supply to maintain bone height. In thinner bone that is subjected to inflammation, adequate blood supply may not be present to maintain its viability. Where gingiva is thin or absent at the time of implant placement, strong consideration must be given to proactively grafting these sites with soft tissue just prior to restoration or at the earliest signs of inflammation to protect the underlying bone. Figure 8 through Figure 12 highlight such a situation.
Loosening/Poor Fit of Prosthetic Components
Micromotion can occur at the interface of the abutment and dental implant platform for several reasons. First, incomplete seating of the abutment can leave a gap and fail to provide adequate clamping force. Second, micromotion could be related to loosening of the screw securing the abutment to the implant, which may result from excessive occlusal force or inadequate torque being provided to fully tighten the screw. Third, this may be the result of using aftermarket parts or trying to mix and match between systems. In all three instances, the lack of intimate fit predisposes the site to micromotion, leading to inflammation and potential for marginal bone loss.
An abutment fitting poorly to the implant platform can facilitate plaque accumulation due to the nature of the margin being open. A number of articles have discussed the benefit of keeping the interface between the implant platform and the abutment as free of plaque accumulation as possible. In fact, this is the general thought process behind platform switching. Anything that hinders this by enhancing inflammation will be detrimental to the health of the implant. The easiest way to avoid this is to expose a radiograph at the time of prosthesis insertion and periodically thereafter to verify a well-fitting abutment/implant platform interface.
Screw loosening has historically been a common complication seen with dental implants.25 A screw may begin to loosen for various reasons, including inadequate torque being applied for clamping force as well as reuse of the final screw between the laboratory and clinician as the prosthesis is checked numerous times for fit and occlusion. Also, multiple tightenings of the screw can change its dimensions, diminishing its clamping ability. Other reasons for screw loosening include use of screws made by companies other than the original manufacturer or that are meant for a provisional and not the final restoration, or excess occlusal force where the screw is the “weak link” absorbing excessive forces. Clinicians must make sure their torque drivers continue to be accurate,26 to periodically check occlusion because it can change,27 and to consider using an ablative shield such as a nightguard to diminish forces on the implant.
Finally, the mixing and matching of aftermarket restorative parts has been a growing problem. As dental implantology has become a burgeoning business, an increasing number of companies have entered into the market to capitalize on the opportunity to enhance either the ease of restoration or final esthetics. Studies, including work by Gigandet et al,28 are continuing to emerge that demonstrate original manufacturer parts fit better than other brands. The present authors suggest that clinicians require their laboratories to use parts from the original manufacturer for a dental implant restoration.
Presence of Foreign Materials
Foreign materials on either the abutment/dental implant surface or in the immediately surrounding tissue can act as a nidus for inflammation. Articles29,30 have focused on the adverse effect of dental cement not being completely removed at the time of crown placement. Linkevicius et al31 demonstrated that the probability of leaving cement behind relates to the depth of the restoration below the soft tissues. Moreover, an adverse synergy may exist for patients who have both residual cement and a history of periodontitis.32 This points to the inflammatory imbalance that these two factors may play.
Further complicating the use of dental cement is that many of the materials used are radiolucent and go undetected by dental radiographs.33 While the easy solution would be to avoid cementing any restorations, the positioning of the dental implant may make screw retention difficult or expensive. Wadhwani and Piñeyro34 discussed strategies to circumvent this problem. (Note: This topic is also discussed by Link-Bindo et al in “Common Prosthetic Implant Complications in Fixed Restorations” on page 431 in this issue.)
Titanium particles are another foreign material that has been found in the soft tissues surrounding a dental implant and are associated with peri-implant inflammation.29,35 How these particles become located in this region, what problems they present, and how they should best be handled are all issues that must be addressed, but how to best do this can only be conjectured at this time. The presence of titanium particles could possibly be due to part of the dental implant surface material (coating or residual blast material) coming off at the time of placement, oral-hygiene measures damaging the surface, or possibly wear of the materials at the interface between the abutment and implant platform, similar to what has been reported in the orthopedic literature with joint replacement.36
Just how detrimental these particles may be is controversial; however, Wilson et al35 demonstrated their presence in soft tissues surrounding peri-implant disease. The presence of this foreign material may also help to explain why many of the approaches used for peri-implant mucositis fail to render the sites free from inflammation. Further study is warranted to better determine the cause-and-effect relationship of this material. An approach that seeks to ablate this material, such as using a laser, may be warranted when treating inflammation around a dental implant.
Reuse of Healing Abutments
Maintaining an environment that is free from inflammation during the healing period following implant placement and beyond is an emerging area of concern. Since the introduction of dental implants by Per-Ingvar Brånemark and colleagues, clinicians have pushed to allow for the healing of the implant in a transgingival manner rather than to have it submerged. To limit inflammation, oral-hygiene measures have included a variety of instruments for professional cleaning, platform switching, the use of chlorhexidine, and the administration of systemic or locally delivered antimicrobial agents.
Nevertheless, these strategies can be limited if healing abutments are being reused. At face value, clinicians may believe that their anti-infective measures of surface decontaminants and autoclaving are ridding the abutment of all bacterial contamination. While this may be so, these approaches do not eliminate residual proteinaceous materials present on the abutment’s surface. Wadhwani et al37 recently reported that this is indeed the case; they purposefully disinfected abutments using various techniques and subsequently stained them for residual protein, which was present in all cases (Figure 13 and Figure 14). The question of whether the presence of these materials potentially led to crestal bone loss beyond physiologic modeling/remodeling, thereby exposing the roughened surface of the dental implant, is an area of emerging investigation. Clearly, reusing parts may not be in the best interest of the implant patient.
Cardiovascular Disease
While no definitive conclusions can be made at this time, studies are emerging that may point to cardiovascular disease as an indirect contributor to peri-implant disease, as patients with this condition may have a profile suggestive that they are prone to inflammation.38,39 If this is indeed an inflammatory risk factor of significance, avoiding cement-retained restorations on dental implants in favor of screw-retained restorations may be one strategy with such patients to reduce this potential risk. In addition, increasing the frequency of maintenance may help avoid the adverse risks that are present in this patient group.
Conclusion
Since the aforementioned 2006 European periodontal workshop, a number of emerging risks to peri-implant disease have continued to be identified. The fundamental theme appears to center on an inflammatory dysbalance, which is the authors’ term. It refers to the body’s inability to contain inflammation, thus becoming overwhelmed, leading to breakdown around the dental implant. Identifying these potential risks and employing effective preventive or treatment strategies are solutions to avoiding biologic complications that lead to dental implant problems. As implants continue to be increasingly used and researched, dentistry’s understanding of this important and evolving area will be enhanced.
Acknowledgments
Figure 5 through Figure 7 are courtesy of Gonzalo Blasi Beriain, DDS, of University of Maryland Dental School in Baltimore, Maryland. Figure 13 and Figure 14 are courtesy of Chandur Wadhwani, DDS, of Bellevue, Washington.
Disclosure
The authors had no disclosures to report.
About the Authors
Paul S. Rosen, DMD, MS
Baltimore College of Dental Surgery
University of Maryland Dental School
Baltimore, Maryland
Private Practice
Yardley, Pennsylvania
Stuart J. Froum, DDS
Clinical Professor
Department of Periodontology and Implant Dentistry
Kriser Dental Center
New York University Dental School
New York, New York
Private Practice
New York, New York
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282-285.
2. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63-76.
3. Derks J, Schaller D, Håkansson J, et al. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95(1):43-49.
4. Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81(2):231-238.
5. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):32(5):533-540.
6. Decker AM, Sheridan R, Lin GH, et al. A prognosis system for periimplant diseases. Implant Dent. 2015;24(4):416-421.
7. Heitz-Mayfield LJ, Salvi GE, Botticelli D, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22(3):237-241.
8. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
9. Serino G, Turri A. Outcome of surgical treatment of peri-implantitis: results from a 2-year prospective clinical study in humans. Clin Oral Implants Res. 2011;22(11):1214-1220.
10. Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res. 2014;25(1):132-136.
11. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
12. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863.
13. Brown IS. Current advances in the use of lasers in periodontal therapy: a laser-assisted new attachment procedure case series. Clinic Adv Periodontics. 2013;3(2):96-104.
14. Fletcher P, Constantinides C. Resolution of a peri-implantitis defect using sterile saline for implant surface detoxification: a case report with clinical re-entry. Clinic Adv Periodontics. 2015;5(4):235-241.
15. Yoshino T, Yamamoto A, Ono Y. Innovative regeneration technology to solve peri-implantitis by Er:YAG laser based on the microbiologic diagnosis: a case series. Int J Periodontics Restorative Dent. 2015;35(1):67-73.
16. Heitz-Mayfield LJ, Mobelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29 suppl:325-345.
17. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041.
18. Hermann JS, Buser D, Schenk RK, et al. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000;11(1):1-11.
19. Linkevicius T, Apse P. Biologic width around implants. An evidence-based review. Stomatologija. 2008;10(1):27-35.
20. Salama H, Salama MA, Garber D, Adar P. The interproximal height of bone: a guidepost to predictable aesthetic strategies and soft tissue contours in anterior tooth replacement. Pract Periodontics Aesthet Dent. 1998;10(9):1131-1141.
21. Amarante ES, Chambrone L, Lotufo RF, Lima LA. Early dental plaque formation on toothbrushed titanium implant surfaces. Am J Dent. 2008;21(5):318-322.
22. Apse P, Zarb GA, Schmitt A, Lewis DW. The longitudinal effectiveness of osseointegrated dental implants. The Toronto Study: peri-implant mucosal response. Int J Periodontics Restorative Dent. 1991;11(2):94-111.
23. Lin GH, Chan HL, Wang HL. Effects of currently available surgical and restorative interventions on reducing midfacial mucosal recession of immediately placed single-tooth implants: a systematic review. J Periodontol. 2014;85(1):92-102.
24. Gobbato L, Avila-Ortiz G, Sohrabi K, et al. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implants. 2013;28(6):1536-1545.
25. Papaspyridakos P, Chen CJ, Chuang SK, et al. A systematic review of biologic and technical complications with fixed implant rehabilitations for edentulous patients. Int J Oral Maxillofac Implants. 2012;27(1):102-110.
26. Neugebauer J, Scheer M, Mischkowski RA, et al. Comparison of torque measurements and clinical handling of various surgical motors. Int J Oral Maxillofac Implants. 2009;24(3):469-476.
27. Bergmann RH. Occlusal considerations for dental implant restorations. Compend Contin Educ Dent. 2014;35(7):455-458.
28. Gigandet M, Bigolin G, Faoro F, et al. Implants with original and non-original abutment connections. Clin Implant Dent Relat Res. 2014;16(2):303-311.
29. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontal. 2009;80(9):1388-1392.
30. Linkevicius T, Vindasiute E, Puisys A, Peciuliene V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin Oral Implants Res. 2011;22(12):1379-1384.
31. Linkevicius T, Vindasiute E, Puisys A, et al. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin Oral Implants Res. 2013;24(1):71-76.
32. Linkevicius T, Puisys A, Vindasiute E, et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24(11):1179-1184.
33. Wadhwani C, Hess T, Faber T, et al. A descriptive study of the radiographic density of implant restorative cements. J Prosth Dent. 2010;103(5):295-302.
34. Wadhwani C, Piñeyro A. Technique for controlling the cement for an implant crown. J Prosthet Dent. 2009;102(1):57-58.
35. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15.
36. Sakamoto M, Watanabe H, Higashi H, Kubosawa H. Pseudotumor caused by titanium particles from a total hip prosthesis. Orthopedics. 2016;39(1):e162-e165.
37. Wadhwani C, Schonnenbaum TR, Audia F, Chung KH. In-vitro study of the contamination remaining on used healing abutments after cleaning and sterilizing in dental practice. Clin Implant Dent Relat Res. 2015 Dec 7. doi: 10.1111/cid.12385.
38. Renvert S, Aghazadeh A, Hallström H, Persson GR. Factors related to peri-implantitis - a retrospective study. Clin Oral Implants Res. 2014;25(4):522-529.
39. Saaby M, Karring E, Schou S, Isidor F. Factors influencing severity of peri-implantitis. Clin Oral Implants Res. 2016;27(1):7-12.