You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Implant dentistry continues to gain popularity among practitioners and patients as a preferred treatment option to replace missing teeth. It is imperative that the dental team (surgeon, restorative dentist, and dental hygienist) work in partnership with patients to continually maintain peri-implant health. This is particularly true during the maintenance phase of implant therapy when the dental team initiates a strict maintenance protocol to monitor and maintain peri-implant health. The majority of biological and prosthetic complications that can cause implant failure can be identified or corrected early in the process, so careful and regular monitoring of peri-implant health is imperative. This includes periodic clinical and radiographic monitoring of peri-implant tissues and restorations. This paper is a culmination of contemporary clinical research and guidance available from the body of literature, and position papers/statements from the American Academy of Periodontology, American College of Prosthodontists, American Academy of General Dentistry, and the American Association of Oral and Maxillofacial Surgeons. The intent is to provide key information regarding assessment of peri-implant tissue health and to provide the practitioner with an algorithm for treatment when need arises.
Several short- and long-term studies have shown implant survival rates as high as 92%.1 It is important for dental clinicians to recognize the difference between implant survival and implant success. Implant survival describes an implant that is retained in the mouth after placement. This includes implants surrounded by inflamed, diseased tissues and those with substantial bone loss. When prosthetic or biological complications arise, it is imperative that treatment is administered without delay.
Implant success is based on several criteria that have been reported in the past, such as absence of mucosal inflammation, lack of mobility, and radiographic evidence of bone loss less than 1 mm after abutment connection and 0.2 mm thereafter.2,3 Advances in material science and surface modifications, implant design, abutment-implant interface, and concepts such as platform switching necessitate reinforcement of clinical evaluation, diagnosis, and treatment planning to maintain existing dental implants.
Clinical Evaluation
Based on the available evidence, proper diagnosis of implant health and disease requires clinical evaluation of the following parameters at the time of implant restoration and each maintenance appointment:
• Soft-tissue evaluation: color, contour, consistency, texture
• Clinical probing depth
• Bleeding on probing
• Radiographic evaluation and/or update
• Presence/absence of suppuration
• Location of the free gingival margin
• Presence/absence of keratinized tissue
• Mobility
Peri-Implant Soft-Tissue Evaluation
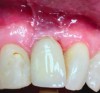
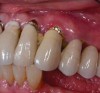
The mucosa surrounding the implant should be evaluated for clinical signs of inflammation such as redness, erythema, and inflammation, which can be assessed by bleeding on probing (Figure 1).4,5 Several gingival indices have been modified for their application around implants.6,7 Difficulties in recording mucosal inflammation have been reported, such as non-keratinized peri-implant mucosa normally appearing redder in color than keratinized tissue.8,9
Clinical Probing Depth
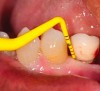
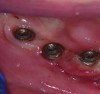
Peri-implant probing with a force of 0.25 Ncm at regular intervals is important to identify the development of peri-implant disease as well as its progression.1,10 A baseline probing depth should be recorded at the time of installation of the restoration relative to a fixed landmark on the implant or its superstructure (Figure 2).1,4 An increase in probing depth would warrant further evaluation by means of radiographs to determine the presence of bone loss surrounding the implant.1 Concerns regarding probing causing damage to the peri-implant tissue have been raised in the past; however, recent literature shows the tissue to heal within five days.10 Probing depths may vary depending on the depth of implant placement, contour of the implant, superstructure, and probing force. Hence, the initial probing depth itself should not be used as a diagnostic tool for diagnosing health and peri-implant disease, but an increase in depth over time suggests a diseased environment and the need for further evaluation.
Bleeding on Probing
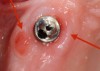
Gentle probing resulting in bleeding suggests the presence of inflammation in response to a biofilm and/or other factors (Figure 3). A study by Lang et al showed a high negative predictive value for bleeding on probing around implants.11 There was no bleeding on probing around healthy peri-implant tissues, whereas there was a high percentage of bleeding around sites that were affected by disease. This is in contrast to reports noting that increased probing force is the possible cause of bleeding on probing, leading to its inaccuracy in determining the presence of disease.12 Another study showed bleeding on probing around implants to be of higher diagnostic accuracy and a better predictor of disease than bleeding on probing around teeth.13
Radiographs
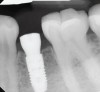
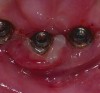
Radiographs taken at time of implant placement (Figure 4) and at the time of prosthesis installation (Figure 5) should serve as baseline radiographs.4,14,15 If clinical signs suggest the presence of peri-implantitis, a new periapical or bitewing radiograph of the site should be taken and compared with baseline to confirm or negate the initial diagnosis. Radiographs have limitations when bone loss presents on the buccal or lingual of the implant.15 Careful consideration is warranted when determining the difference between adaptive bone remodeling and creation of the supra-crestal attached tissues (biologic width) that occurs around non-platform switched implants, as well as bone loss with the presence of clinical signs of inflammation due to disease.
Suppuration
Suppuration may be attributed to a change in the pathologic environment within the sulcus surrounding the implant and is commonly associated with peri-implant disease and bone loss (Figure 6).4,8,13,14,16-18 A study by Sanz et al19 showed 65% of the connective tissue being replaced by an inflammatory infiltrate in the peri-implant infection group, compared with 8% in the non-infection group. This finding has been substantiated by various other studies that have shown an increase in the number of plasma cells, macrophages and lymphocytes surrounding peri-implantitis lesions. Based on these findings, the presence of suppuration may be attributed to the large inflammatory infiltrate occupying the connective tissue surrounding peri-implantitis sites.
Location of the Free Gingival Margin
Clinical attachment levels should be evaluated at the time of probing depth measurement (Figure 7). Similar to teeth, the soft-tissue margin around implants should be determined relative to a fixed reference point on the implant or the implant superstructure.8,13 The progressive apical migration of the soft-tissue margin signifies a loss of clinical attachment around the implant and the need for further evaluation.
Presence or Absence of Keratinized Tissue
The keratinized tissue is measured from the gingival margin around the implant to the muco-gingival junction (Figure 8). Historically, an adequate zone of keratinized tissue was defined as 2 or more mm to maintain gingival health.20 Until now, the consensus remains divided on the importance of keratinized tissue surrounding implants. While some studies show implants with a greater amount of keratinized tissue had better plaque and gingival indices in addition to reduced alveolar bone loss surrounding the implant, opponents maintain there is no association between the lack of keratinized tissue and peri-implant bone loss despite greater plaque accumulation and gingival inflammation.17,21,22 Although the evidence is conflicting, readers are encouraged to keep in mind the different orientation of the gingival fibers around teeth and implants. Around natural teeth, the gingival fibers run perpendicular to the long axis attaching to the tooth surface, whereas around implants the fibers run parallel to the long axis of the implant and do not attach to the implant surface, providing a weaker mechanical barrier to plaque.
Mobility
Mobility of the implant should be assessed along with other clinical parameters to rule out implant failure (Figure 9). A mobile implant has a hopeless prognosis due either to lack of osseointegration or severity of peri-implant bone loss. This should not be confused with prosthetic failure such as screw loosening within the superstructure.13
Arriving at a Diagnosis and Treatment Planning
A recent consensus report published by the American Academy of Periodontology has provided a better understanding of peri-implant disease.23
Erythema, bleeding on probing, swelling, and suppuration are absent in the peri-implant tissues in health. In the presence of disease, the soft tissue around the implant is characterized by clinical signs of inflammation, increased probing depths, swelling, and suppuration, with the only differentiating factor between peri-mucositis and peri-implantitis being the presence of progressive radiographic bone loss compared to baseline.
Several treatment options have been proposed in the literature concerning the treatment of peri-implant disease such as hand instrumentation,24,25 open flap debridement,26,27 resective therapy,28,29 regenerative therapy,30,31 implantoplasty,32 and lasers.33
While evidence has shown non-surgical to be very effective in the presence of peri-implant mucositis (Figure 10), in the presence of bone loss, a surgical approach has been shown to have a better outcome. The surgical treatment option to be undertaken depends on various factors, such as the defect configuration, defect size, implant surface, implant position, and disease-causing etiology requiring a referral to the care of a specialist.
Table I describes the clinical, radiographic features, and recommended treatment options.14,23,34
The flow chart (Figure 11) details clinical and radiographic examination of peri-implant tissues and recommended treatment when indicated.14,34,35
Conclusion
While an estimated 100,000 to 300,000 dental implants are placed every year, the literature suggests a high frequency of peri-implant mucositis around 30.7% of implants, and peri-implantitis around 9.6% of implants.36 Within the limitations of this short report, it can be concluded that all existing implants should be continually monitored by a thorough soft-tissue and radiographic evaluation to prevent any biological and prosthetic complications that may arise.
Therefore, the authors recommend that healthy implants and implants with peri-mucositis be treated and maintained by the dental clinicians, while a diagnosis of peri-implantitis needs a referral to the specialist for further evaluation and treatment.
References
1. Mishler OP, Shiau HJ. Management of peri-implant disease: a current appraisal. J Evid Based Dent Pract. 2014;14S:53-59.
2. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
3. Sennerby L, Becker W. Implant success versus survival. Clin Implant Dent Relat Res. 2000;2(3):119.
4. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(2):533-540.
5. Lang NP, Berglundh T; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(suppl 11):178-181.
6. Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6 suppl):610-616.
7. Apse P, Zarb GA, Schmitt A, Lewis DW. The longitudinal effectiveness of osseointegrated dental implants. The Toronto Study: peri-implant mucosal response. Int J Periodontics Restorative Dent. 1991;11(2):94-111.
8. Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants. 2004;19(suppl):116-127.
9. Listgarten MA, Lang NP, Schroeder HE, Schroeder A. Periodontal tissues and their counterparts around endosseous implants. Clin Oral Implants Res. 1991;2(3):1-19.
10. Gerber JA, Tan WC, Balmer TE, et al. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin Oral Implants Res. 2009;20(1):75-78.
11. Lang NP, Wetzel AC, Stich H, Caffesse RG. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res. 1994;5(4):191-201.
12. Etter TH, Håkanson I, Lang NP, et al. Healing after standardized clinical probing of the periimplant soft tissue seal: a histomorphometric study in dogs. Clin Oral Implants Res. 2002;13(6):571-580.
13. Kim Y, Oh TJ, Misch CE, Wang HL. Occlusal considerations in implant therapy: clinical guidelines with biomechanical rationale. Clin Oral Implants Res. 2005;16(1):26-35.
14. Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri‐implantitis: managing peri‐implant mucositis. J Clin Periodontol. 2015;42(suppl 16):S152-S157.
15. Alani A, Kelleher M, Bishop K. Peri-implantitis. Part 1: Scope of the problem. Br Dent J. 2014;217(6):281-287.
16. Padial-Molina M, Suarez F, Rios HF, et al. Guidelines for the diagnosis and treatment of peri-implant diseases. Int J Periodontics Restorative Dent. 2014;34(6):e102-e111.
17. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33(4):296-301.
18. Fransson C, Wennström J, Berglundh T. Clinical characteristics at implants with a history of progressive bone loss. Clin Oral Implants Res. 2008;19(2):142-147.
19. Sanz M, Alandez J, Lazaro P, et al. Histo-pathologic characteristics of peri-implant soft tissues in Brånemark implants with 2 distinct clinical and radiological patterns. Clin Oral Implants Res. 1991;2(3):128-134.
20. Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623-627.
21. Yuan JC, Sukotjo C. Occlusion for implant-supported fixed dental prostheses in partially edentulous patients: a literature review and current concepts. J Periodontal Implant Sci. 2013;43(2):51-57.
22. Chung DM, Oh TJ, Shotwell JL, et al. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol. 2006;77(8):1410-1420.
23. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. J Clin Periodontol. 2018;45(suppl 20):S286-S291.
24. Suárez-López Del Amo F, Yu SH, Wang HL. Non-surgical therapy for peri-implant diseases: a systematic review. J Oral Maxillofac Res. 2016;7(3):e13.
25. Heitz‐Mayfield LJ, Salvi GE, Botticelli D, et al; Implant Complication Research Group. Anti‐infective treatment of peri‐implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22(3):237-241.
26. Hallström H, Persson GR, Lindgren S, Renvert S. Open flap debridement of peri‐implantitis with or without adjunctive systemic antibiotics: a randomized clinical trial. J Clin Periodontol. 2017;44(12):1285-1293.
27. Albaker AM, ArRejaie AS, Alrabiah M, et al. Effect of antimicrobial photodynamic therapy in open flap debridement in the treatment of peri-implantitis: a randomized controlled trial. Photodiagnosis Photodyn Ther. 2018;23:71-74.
28. Romeo E, Lops D, Chiapasco M, et al. Therapy of peri‐implantitis with resective surgery. A 3‐year clinical trial on rough screw‐shaped oral implants. Part II: radiographic outcome. Clin Oral Implants Res. 2007;18(2):179-187.
29. Englezos E. Resective treatment of peri‐implantitis. Clinical and radiographic outcome after 2 years. Clin Oral Implants Res. 2018;29(S17):378-378.
30. Romanos GE, Nentwig GH. Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. Int J Periodontics Restorative Dent. 2008;28(3):245-255.
31. Jepsen K, Jepsen S, Laine ML, et al. Reconstruction of peri-implant osseous defects: a multicenter randomized trial. J Dent Res.2016;95(1):58-66.
32. Bianchini MA, Galarraga‐Vinueza ME, Apaza‐Bedoya, K, et al. Two to six‐year disease resolution and marginal bone stability rates of a modified resective‐implantoplasty therapy in 32 peri‐implantitis cases. Clin Implant Dent Relat Res. 2019; doi: 10.1111/cid.12773.
33. Nevins M, Nevins ML, Yamamoto A, et al. Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int J Periodontics Restorative Dent. 2014;34(4): 461-466.
34. Bidra AS, Daubert DM, Garcia LT, et al. Clinical practice guidelines for recall and maintenance of patients with tooth-born and implant-borne dental restorations. J Prosthodont. 2016;25(suppl 1):S32-S40.
35. Romanos GE, Javed F, Delgado-Ruiz RA, Calvo-Guirado JL. Peri-implant diseases: a review of treatment interventions. Dent Clin North Am. 2015;59(1):157-178.
36. Atieh MA, Alsabeeha NH, Faggion CM, Duncan WJ. The frequency of peri‐implant diseases: a systematic review and meta‐analysis. J Periodontol. 2013;84(11):1586-1598