You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
A recent study determined that 39.1% of single-unit crowns were bonded with a resin cement.1 Not every ceramic crown needs to be adhesively bonded, but bonding should be used if the clinician desires to increase retention of the crown or bond the crown to the tooth to reinforce its strength. The steps for bonding ceramic crowns are straightforward in concept; however, the options can become confusing due to the many different products available to achieve the basic goals of adhesive bonding. Additionally, the treatment of the surface of the crown must be coordinated between the dental laboratory and the clinician, which can affect the sequence of the procedure. Bonding a crown is also nuanced by the slight differences in procedure for glass-containing ceramics and zirconia. This review aims to provide a step-by-step recipe for bonding to glass-containing ceramics and zirconia.
Surface Treatment
The process of bonding a ceramic crown usually starts in the dental laboratory. The goal in the laboratory is to achieve surface texture on the intaglio surface of the crown to gain micromechanical retention. The clinician has the option to treat the intaglio surface of the crowns within their clinic; however, this request should be given to the dental laboratory because many laboratories will default to performing their own surface treatment. Either way, clinicians should contact their laboratory to determine how their ceramic crowns are being treated before being sent to the office.
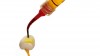
For glass-containing ceramics, micromechanical retention may be achieved by selectively removing glass from the surface of the crown with hydrofluoric acid (Figure 1). Glass-ceramics include feldspathic porcelain, leucite-reinforced glass-ceramic, and lithium disilicate-based ceramics. Hydrofluoric acid is typically used in 5% and 9.5% to 10% concentrations. Figure 2 through Figure 5 show the etch pattern of lithium disilicate etched with 5% and 9.5% hydrofluoric acid for 20 seconds or 60 seconds. The concentration of the hydrofluoric acid had less impact on the etch pattern than the amount of time for which the ceramic was etched. Each ceramic should be etched for the amount of time recommended by its manufacturer, but generalizations will be presented. Feldspathic porcelain typically requires 2 minutes of etching with hydrofluoric acid. Leucite-reinforced glass-ceramic requires 1 minute of etching. Lithium disilicate materials will generally require between 20 and 30 seconds of etching. After etching a glass-ceramic crown, the intaglio surface of the crown will have a frosted appearance (Figure 6).
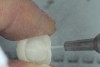
Zirconia, on the other hand, does not contain any glass, and therefore it is unaffected by hydrofluoric acid. Surface treatment of zirconia must be performed by airborne particle abrasion (sandblasting) with alumina particles (Figure 7). Fifty-micron (µm) alumina particles are typically used, whereas glass beads are ineffective on the surface of zirconia. Figure 8 and Figure 9 show the surface of zirconia after sandblasting with alumina and glass, respectively. Sandblasting is performed from a distance of 10 mm from the surface of the crown with protection of the margins and external surface of the crown. The pressure used during sandblasting should be 2 to 2.5 bar for typical 3 mol% yttria-containing zirconia.2 For the newer 4 or 5 mol% yttria-containing translucent zirconia, which is incapable of transformation toughening, the sandblasting pressure may be reduced 1 bar in order to best preserve the strength of the material.3
Cleaning
After the crown is sent back from the laboratory, it will need to be tried into the patient's mouth to verify clinical parameters such as its margins, contacts, and esthetics. During this try-in process, the crown will more than likely be exposed to saliva, blood, and try-in pastes. These contaminants need to be removed to bond to a clean surface of the ceramic. Several options are available for cleaning a crown after contamination, such as phosphoric acid, ceramic cleaning solutions, sodium hypochlorite, and re-sandblasting.
Phosphoric Acid
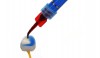
37% phosphoric acid is an effective method for removing contaminants from the surface of glass-containing crowns (Figure 10).4,5 Additionally, phosphoric acid has been suggested by manufacturers to remove salts that are left on the surface of a glass-containing crown after etching with hydrofluoric acid.6 Others have recommended removing these salts with alcohol, ultrasonic cleaning, or steam cleaning. Phosphoric acid, however, cannot be used to clean the surface of zirconia before bonding.7-10 Phosphoric acid will bind to the sites on zirconia that are needed for chemical bonding with the ceramic primer.7
Ceramic Cleaning Solutions
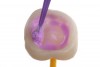
There are ceramic cleaning solutions, such as Ivoclean® (Ivoclar Vivadent, ivoclarvivadent.us) or ZirClean® (Bisco, bisco.com), that can be used for both glass-containing crowns and zirconia (Figure 11).5,8,10-12 These cleaning solutions are composed of saturated zirconia solutions that can bind to salivary contaminants. After application, the solution (with bound contaminants) is rinsed off the crown.
Sodium Hypochlorite
Sodium hypochlorite (or bleach) has been suggested to be used as a cleaning solution for zirconia because there are several studies that have shown its effectiveness for cleaning it.9-11 The mechanism by which bleach cleans has not been proven, but it may be related to the basic pH of the solution.
Sandblasting
Zirconia can be cleaned by re-sandblasting the surface of the zirconia crown with alumina particles.9,10,12,13 Glass-containing ceramics should not be sandblasted after etching. Sandblasting with alumina particles or glass beads will destroy the etch pattern created with hydrofluoric acid etching (Figure 12 and Figure 13). Additionally, sandblasting with alumina may create cracks in glass-ceramic and reduce its strength.14
If the clinician chooses to perform surface treatment (hydrofluoric acid etching or sandblasting) in the clinic, the surface treatment may be performed after try-in of the crown. Therefore, it is no longer necessary to "clean" the crown because the surface treatment will remove contaminants.
Chemical Treatment
A chemical bond can be formed between glass-containing ceramics (feldspathic porcelain, leucite-reinforced glass-ceramic, and lithium disilicate-based ceramics) and resin cement using a solution containing silane (Figure 14). Silane can be obtained in several different types of primers. Pure silane in water and ethanol is available in one-bottle and two-bottle systems. In a two-bottle system, one bottle contains unhydrolyzed silane and the second bottle contains acetic acid/water. When the contents of the bottles are combined, the silane becomes hydrolyzed and activated. A one-bottle silane system contains prehydrolyzed (activated) silane. Prehydrolyzed silane can react with itself over time, and therefore one-bottle silanes have a shorter shelf-life than two-bottle systems. Silane can also be obtained within universal bonding agents; however, several studies have shown that silane incorporated within a universal bonding agent is an ineffective primer for bonding to glass-containing ceramics.15,16
Bonding to zirconia can be achieved using the molecule 10-methacryloyloxydecyl-dihydrogen-phosphate (MDP). This molecule has been proven to bond with zirconia through several different chemical analyses.17-19 There are solutions of pure MDP (eg, Z-Prime™ Plus, Bisco). Additionally, the MDP that is incorporated in universal bonding agents has been shown to be effective for bonding to zirconia. Finally, there are ceramic primers that contain both silane and MDP (eg, Clearfil® Ceramic Primer, Kuraray, kuraraydental.com; and Monobond® Plus, Ivoclar Vivadent) that can be used as a primer on both zirconia and glass-containing ceramics.
Cement Selection
After surface treating, cleaning, and chemically preparing the ceramic restoration, a resin cement must be selected to complete adhesive bonding. Generally, resin cements can be divided into those that are used with a primer on the tooth (conventional resin cements) and those used without a tooth primer (self-adhesive resin cements). The choice between these two types of cements can be made based on the amount of time that isolation can be achieved and the amount of retention needed.
Considerations With Achieving Isolation
Achieving isolation for cementation of an indirect restoration may be difficult. If all margins of the restoration are accessible (such as with some onlays/inlays), a rubber dam may be used. For most cases, other methods to achieve isolation that do not require access to margins of the crown can be used, including cheek/lip retractors (eg, Optragate®, Ivoclar Vivadent), retractors with built-in suction (Isovac®, Zyris, zyris.com), or stiff cardboard cheek retractors placed in the lingual and buccal vestibule.
Often, maintaining isolation is challenging. The amount of time for which isolation may be achieved can be a deciding factor when choosing a type of resin cement. A self-adhesive resin cement that does not require application of an adhesive on the tooth or light-curing the adhesive on the tooth may be a better clinical decision if isolation is difficult to maintain. There is limited research regarding the amount of time for which isolation should be maintained after the crown has been seated. In theory, the cement at the internal surface of the crown is sealed from oral fluids after the crown is seated. Cement at the margin of the crown may be prematurely exposed to blood or saliva if the patient is asked to close the mouth to confirm occlusion or if excess cement removal induces bleeding. As a reference for the clinician who is managing the time for isolation, bond-strength development for dental resins proceeds slowly (several minutes) for a chemical curing and quickly (within seconds) for light-initiated curing.20 Bond development will occur quickly at the surface being cured; therefore, even tack-curing cement at the margins for 2 to 3 seconds before salivary contamination may allow up to 50% of the bond strength to be achieved.21
Considerations With Achieving Retention
The advantage of using a conventional resin cement with a tooth primer is that these cements provide greater retention than self-adhesive resin cements.22 In the clinical experience of this author, debonded ceramic crowns more frequently have remnant cement on the intaglio surface of the crown, implying a lesser bond to the tooth preparation than the intaglio of the crown. Therefore, crown preparations with compromised retention will benefit from the additional clinical step of primer application on the tooth (Figure 15).
Considerations With Light-Curing Cement
With the use of dual-cure cements, the clinician has the option of light-curing the cement to initiate its polymerization. Light-curing the margins will help improve the wear resistance of the cement; however, the opacity of a crown will limit the amount of light that will reach the underlying cement (Figure 16). Laboratory studies have reported that curing light will pass through traditional zirconia (3 mol% yttria) restorations, albeit with an irradiance that decreases significantly with darker shades and thicker restorations.23 The study suggested that shade A1 or B1 zirconia benefits from light polymerization up to 1.5 mm in thickness, whereas darker shades benefited only up to 0.5 mm in thickness. Another recent study reported that resin cement can be cured through 2 mm of monolithic translucent zirconia (5 mol% yttria) and 2.5 mm of lithium disilicate.24
Summary
A step-by-step guide for bonding a crown based on the information in this article is summarized in Table 1 and Table 2. The first table describes the steps if the clinician receives the crown from the laboratory with surface treatment already performed, whereas the second table describes the steps performed if surface treatment is performed within the clinic.
About the Author
Nathaniel C. Lawson, DMD, PhD, is assistant professor, Department of Clinical and Community Sciences, Division of Biomaterials, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
References
1. Lawson NC, Litaker MS, Ferracane JL, et al; National Dental PBRN Collaborative Group. Choice of cement for single-unit crowns: findings from the National Dental Practice-Based Research Network. J Am Dent Assoc. 2019; pii: S0002-8177(19)30038-8. doi: 10.1016/j.adaj.2019.01.021.
2. Okada M, Taketa H, Torii Y, et al. Optimal sandblasting conditions for conventional-type yttria-stabilized tetragonal zirconia polycrystals. Dent Mater. 2019;35(1):169-175.
3. Fu CC, Darkoue Y, Burgess JO, et al. Surface treatment - shear bond strength and biaxial flexural strength of zirconia. J Dent Res. 98 (spec iss A); 2019 [abstract 3619].
4. Lyann SK, Takagaki T, Nikaido T, et al. Efficacy of various surface treatments on the bonding performance of saliva-contaminated lithium-disilicate ceramics. J Adhes Dent. 2019;21(1):51-58.
5. Borges ALS, Posritong S, Özcan M, et al. Can cleansing regimens effectively eliminate saliva contamination from lithium disilicate ceramic surface? Eur J Prosthodont Restor Dent. 2017;25(1):9-14.
6. Ultradent Products. https://www.ultradent.com/en-us/Dental-Products-Supplies/Bond-Etch/Etchants/Hydrofluoric-Acid-Gel/Ultradent-Porcelain-Etch-and-Silane-Ceramic-Etchant-and-Silane-Solution/Pages/default.aspx. Accessed May 1, 2016.
7. Phark JH, Duarte S Jr, Kahn H, Blatz MB, Sadan A. Influence of contamination and cleaning on bond strength to modified zirconia. Dent Mater. 2009;25(12):1541-1550.
8. Feitosa SA, Patel D, Borges AL, et al. Effect of cleansing methods on saliva-contaminated zirconia--an evaluation of resin bond durability. Oper Dent. 2015;40(2):163-171.
9. Yoshida K. Influence of cleaning methods on resin bonding to saliva-contaminated zirconia. J Esthet Restor Dent. 2018;30(3):259-264.
10. Kim DH, Son JS, Jeong SH, et al. Efficacy of various cleaning solutions on saliva-contaminated zirconia for improved resin bonding. J Adv Prosthodont. 2015;7(2):85-92.
11. Aladağ A, Elter B, Çömlekoğlu E, et al. Effect of different cleaning regimens on the adhesion of resin to saliva-contaminated ceramics. J Prosthodont. 2015;24(2):136-145.
12. Angkasith P, Burgess JO, Bottino MC, Lawson NC. Cleaning methods for zirconia following salivary contamination. J Prosthodont. 2016;25(5):375-379.
13. Ishii R, Tsujimoto A, Takamizawa T, et al. Influence of surface treatment of contaminated zirconia on surface free energy and resin cement bonding. Dent Mater J. 2015;34(1):91-97.
14. Menees TS, Lawson NC, Beck PR, Burgess JO. Influence of particle abrasion or hydrofluoric acid etching on lithium disilicate flexural strength. J Prosthet Dent. 2014;112(5):1164-1170.
15. Yao C, Yu J, Wang Y, et al. Acidic pH weakens the bonding effectiveness of silane contained in universal adhesives. Dent Mater. 2018;34(5):809-818.
16. Murillo-Gómez F, Rueggeberg FA, De Goes MF. Short- and long-term bond strength between resin cement and glass-ceramic using a silane-containing universal adhesive. Oper Dent. 2017;42(5):514-525.
17. Chen Y, Lu Z, Qian M, et al. Effect of 10-methacryloxydecyl dihydrogen phosphate concentration on chemical coupling of methacrylate resin to yttria-stabilized zirconia. J Adhes Dent. 2017;19(4):349-355.
18. Chuang SF, Kang LL, Liu YC, et al. Effects of silane- and MDP-based primers application orders on zirconia-resin adhesion-A ToF-SIMS study. Dent Mater. 2017;33(8):923-933.
19. Nagaoka N, Yoshihara K, Feitosa VP, et al. Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep. 2017;7:45563.
20. Guo J, Holmes B, Yang B, et al. Determining the temporal development of dentin-composite bond strength during curing. Dent Mater. 2016;32(8):1007-1018.
21. Davidson CL, de Gee AJ, Feilzer A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res. 1984;63(12):1396-1399.
22. Lührs AK, Guhr S, Günay H, Geurtsen W. Shear bond strength of self-adhesive resins compared to resin cements with etch and rinse adhesives to enamel and dentin in vitro. Clin Oral Investig. 2010;14(2):193-199.
23. Ilie N, Stawarczyk B. Quantification of the amount of light passing through zirconia: the effect of material shade, thickness, and curing conditions. J Dent. 2014;42(6):684-690.
24. Turp V, Turkoglu P, Sen D. Influence of monolithic lithium disilicate and zirconia thickness on polymerization efficiency of dual-cure resin cements. J Esthet Restor Dent. 2018;30(4):360-368.