You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Megatrends and demographics are shaping not only who dental patients are but the entire environment and context within which they must be treated. Findings about the relation between not just periodontal treatment but other approaches to controlling systemic inflammation promise to profoundly impact all of healthcare.
New Kinds of Patients in a Transformed Environment
The 65 and over population in the United States, whose health needs profoundly impact practices and patient management, is expanding. In the decades to come, nearly 25% of the population being medically managed will be over the age of 65.
Along with other long-term changes that have intersected to disrupt healthcare, this generation is different than previous ones; they want to live longer and better, they want personalized products and services; and they want to have control over their own health decisions—ie, self-determination.
This population influences and is influenced by megatrends—in consumer behavior, technology, and business—that are coming together to integrate in a fashion that will have a dramatic impact on dentistry and on healthcare in general. Megatrends in technology include powerful information technology, personal engagement technology, and personalization technology.
The business model used to provide healthcare in its current configuration, however, is not sustainable, and has, therefore, ushered in efforts today to more closely integrate medical and oral healthcare, especially now that insurance data indicates that periodontal treatment improves medical outcomes, lowers the cost of treatment, and is, therefore, a “good investment” in terms of creating a model to meet healthcare needs and business realities. To handle the demographic shifts and the cost of healthcare requires a change in the model from treatment to prevention.
Periodontitis is Highly Prevalent
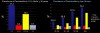
Centers for Disease Control data1 shows the current prevalence of periodontitis among adults over the age of 30 to be close to 50%; of which 10% is severe, 30% moderate, and 10% slight. This overall percentage increases dramatically with age, to the point that it affects 70% of those 65 years of age and older (Figure 1). Further, it affects some ethnicities, including Hispanics—even among younger people—at a significantly higher rate.2 The problem is likely to get worse as the population ages, ultimately dying in their late 70s of heart disease, cancer, and stroke, in order of prevalence.3 This contrasts dramatically with statistics from 1900, when life expectancy was 49.2 years of age and deaths were primarily attributed to infectious diseases including pneumonia, tuberculosis, and dysentery.
The Era of Inflammatory Diseases Has Arrived
We have gone from dying at a relatively young age of acute infection, to living longer and dying of chronic inflammatory diseases of aging. Chronic inflammation is now implicated in many cancers.4 In their study on colon cancer, Sokolosky and Wargovich5 observed the existence of a new era of chronic diseases classified as inflammatory due in part to the influences of modern diets that are increasingly proinflammatory as well as chronic exposure to low levels of environmental toxins. They further noted the impact on epigenetic changes that occur in utero, and the emergence of nutritional therapy in oncology in an effort to decrease the inflammation.5
The explosive increase in type 2 diabetes is closely connected to overeating and obesity throughout much of the world. Inflammation is perhaps the key driving mechanism that links obesity to the metabolic changes characteristic of diabetes.6 Adipose tissue accumulating around the waist increases levels of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1, and interleukin-6, all of which directly contribute to the compromise of pancreatic beta-cell function and the actual loss of beta-cell mass. These mechanisms reduce the insulin response and enhance insulin resistance. Nearly one third of the US population is either prediabetic or diabetic, and 90% of those who are prediabetic are unaware of its existence. Beyond the impact on the health of these individuals with diabetes is its impact on healthcare costs and lost wages in the coming decade—estimated to be $250 billion.
In a recent study, more than 1,000 patients with no prior diagnosis of diabetes were screened in dental practices via a simple hemoglobin (Hb) A1c test and the Diabetes Association Risk Test.7 Investigators found that 35% of the population randomly walking into dental practices had either diabetes or prediabetes. Dental practices can incorporate diabetes screening into their practice using the American Diabetes Association Type 2 Diabetes Risk Test (http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/), which involves seven questions that focus on a family history of diabetes, body mass index, blood pressure, and ethnic background that may confer a higher risk.
Patients with diabetes that is not well controlled are more likely to develop moderate to severe periodontitis8 and may be at increased risk for implant complications.9,10 It is well established that periodontitis, especially moderate to severe periodontitis, adversely affects glycemic control and complications of diabetes.11-14 Most, but not all, clinical trials have shown that treatment of periodontitis significantly reduces HbA1c values, and it is important to control periodontal inflammation to assist in glycemic control.15-17
Because cardiovascular events, including myocardial infarctions and strokes, are strongly associated with elevated chronic inflammation,18 they are also associated with moderate to severe periodontitis, which often increases systemic inflammation.19
Patient Risk Assessment Can Help Enhance Clinical Management of Periodontitis
New paradigms for managing periodontitis do not conform to previous assumptions that all patients are equally susceptible. While bacterial plaque is essential for initiation and progression of periodontitis, not everyone with plaque develops the same severity of periodontitis; patients with similar clinical signs may be on different disease paths.
The recognition that there are risk factors beyond oral hygiene and age marks a major shift in our understanding of periodontitis. Most importantly, we can use the new knowledge to help prevent severe periodontitis and its complications. The most well-validated risk factors for severe periodontitis are smoking, uncontrolled diabetes, and a particular genetic variation that involves an inflammatory gene. In addition, substantial evidence is emerging that obesity is a risk factor for periodontitis development, progression, and less successful response to treatment. All of these risk factors are consistent with the broader findings that individuals with elevated systemic inflammation have increased risk and increased complications with most of the chronic diseases of aging, including periodontitis.
It is important to both measure existing periodontal disease parameters but also to assess risk factors for more severe periodontitis. The small number of validated risk factors allow us to put every patient into a risk category to identify the individuals who are more likely to develop severe periodontitis. Risk classification can then be used to have a more detailed conversation with the patient about the preventive and therapeutic care or specialty referral that may be needed because of their individual risk profile.
Patients can be risk-stratified for more effective periodontitis prevention and treatment in keeping with what is considered a road map for the future of healthcare—P4 medicine20 as described by Leroy Hood, MD, PhD (www.systemsbiology.org). It involves using approaches that are: (1) personalized—based on the path an individual patient is on in terms of disease trajectory; (2) predictive—identifying that path before the disease starts and becomes severe; (3) preventive—extending wellness using proven approaches; (4) participatory—with patient involvement.
Regenerating Hard and Soft Tissue More Predictably
By controlling periodontitis and bone loss in the coming decades, aging populations—who not only want to live longer, they want to live better—seek a return of the function and esthetics that they had at predisease levels. As with procedures like hip replacement, periodontal patients who are missing soft tissue, bone, or teeth want to be regenerated and return to full function and esthetics.
In recent years, periodontal regeneration has become predictable using biologic wound healing enhancements such as platelet-derived growth factor (PDGF).
Soft-tissue capabilities are well documented in terms of what can be predictably achieved in various situations, as described by William Giannobile and Pam McClain21 based on evidence from an American Academy of Periodontology workshop and the consensus reports of five different working groups. They translated their evidence into practical, clinical applications, creating a thought decision tree that can be used by clinicians when seeing patients in their offices. Using this tool to determine the correct management of tissue in a given clinical scenario, it is possible to predictably regenerate many soft-tissue lesions, severe vertical bone defects, and furcation defects. There are also guidelines for identifying patients in whom such treatments are less predictable, and for determining the best approaches for defects that are morphologically different.
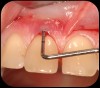
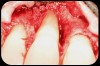
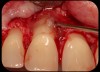
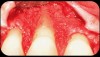
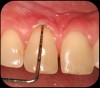
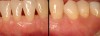
Then, too, are opportunities to prepare the site for regeneration by coupling therapies, applying an enamel matrix derivative, along with traditional bone grafts of demineralized freeze-dried bone allograft; potentially, gains in attachment with minimal probing depth and retained papilla may avoid the need for an implant (Figure 2 through Figure 7).
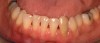
In the coming decades, restorative treatment for aging patients who experience facial growth with recession and erosion below the cemento-enamel junction because of tooth movement may be especially challenging. However, if dentists can employ the tenets of regeneration using autogenous tissues or a cellular dermal matrix combined with enamel matrix derivatives as a regulatory protein, they may predictably achieve large amounts of root coverage with thickened periodontium. This would not eliminate the need for restorative dentistry, but would enable restorative dentists to place restorations that are supragingival and are easily accessible for the patient and the therapist during maintenance (Figure 8 through Figure 10).
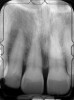
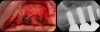
There is also “the Holy Grail” of regeneration, bone morphogenic protein (BMP), whose ability to stimulate the formation of bone where bone does not normally grow was discovered in the mid-60s by orthopedic surgeon Marshall R. Urist, who believed it was destined to bring osteogenesis under the control of surgeons; clinically, this recombinant DNA technology (recombinant human BMPs; rhBMPs) is being used in the regeneration of deficient sites planned for implant placement (Figure 11 through Figure 13).
Inflammation Control
The elimination of inflammation, including smoking cessation, should be a key goal of periodontal therapy, as it perhaps should be for the prevention and treatment of all chronic diseases. The dental community, in particular, will be interested in the final results of the Anti-inflammatory Thrombosis Outcomes Study (CANTOS) study, a placebo-controlled study of more than 17,000 patients being treated with interleukin-1β (IL-1β) inhibitors for the prevention of second heart attacks, as their periodontitis may benefit from the therapy as well.22
Putting it Into Practice
Clinicians can apply their knowledge of risk factors—measuring whatever is convenient to measure—to classify their patients to enhance the prevention and management of severe periodontitis, first by placing each patient into a risk category that guides prevention and monitoring frequencies.
They can regenerate tissue predictably in many situations and monitor inflammation as a key endpoint to be evaluated while integrating with medicine.
Implications of Inflammatory Findings: Coupling Therapies
Clearly all healthcare professionals, including dentists, should be aware of the need to consider inflammation control to improve patient outcomes and response to other treatment and to counsel patients on health behavior management. They may also benefit from new drug development, such as Canakinumab (Ilaris), a human monoclonal antibody against IL-1β, because the presence of cardiovascular disease makes periodontal disease more difficult to manage. The presence and concentration of cytokines, interleukins, C-reactive proteins, and tumor necrosis factors are measurable indicators of patients’ health, so whether they have periodontitis or their arteries are blocked, tests will show the same inflammatory markers, and those with moderate to severe periodontitis who also have cardiovascular disease will make that disease more difficult to manage.23
Given that up to 50% of susceptibility to chronic periodontitis may be due to genetic factors, an effective treatment approach may well involve coupling local and systemic treatment. This approach is supported in the dermatology literature, where the treatment of rosacea combines suppression of the inflammatory response with anti-microbial therapy.24
Dentistry of the Future
This concept of coupling technologies to enhance each is likely to be the future of dentistry, especially as new technologies develop. Clinicians can use advanced fixation devices such as titanium-reinforced membranes and traditional bone allografts along with growth factors combined to stabilize the area, expand the ridge, and stimulate new bone formation.
Currently, the development of nano-biomaterials that increase tissue growth, decrease infection, and inhibit inflammation are creating excitement. They include nano-tubes, whose surfaces are smooth and so small that bacteria cannot penetrate them or attach to them, thereby inhibiting the formation of biofilms, which significantly decreases postoperative and postimplant infection in orthopedics; yet they encourage the attachment of connective tissue cells to the implant surface and bone to the implant surface itself.
Nano-technology is also the basis of nano-diamonds, carbon-based particles used in medicine to image individual cells and individual strands of DNA. They are currently developing nano-biomaterials that increase tissue growth, decrease infection, and inhibit inflammation. The general belief about nanomaterials is that their size provides unique properties, one of which is super-high surface energy likely to interact better with the body and be less vulnerable to infection.
Why is severe periodontitis important? If periodontitis influences other major diseases, it changes dentistry forever.
A better ability to understand risk factors is being driven by major changes in healthcare in general. Perhaps first among these stems from the so-called perio-systemic link—ie, that periodontitis is linked to chronic diseases including diabetes and cardiovascular disease, and further, that its management can improve outcomes and lower treatment costs, as reported in insurance studies, including one by Jeffcoat et al showing that periodontal therapy led to reduced costs, particularly in patients with diabetes, cardiovascular disease, cerebral vascular disease, and rheumatoid arthritis.25
A better ability to understand periodontal disease risk factors and to use effective treatment approaches offers dentistry the opportunity to ride the wave as opposed to developing approaches in isolation that may or may not be incentivized and rewarded more broadly. Medical insurance suddenly has an incentive for promoting and encouraging what is a relatively low-cost approach to reducing cardiovascular events.
The drug industry too may see the value in in developing drugs for the management of periodontal disease due to a proven link to cardiovascular disease, with market potential in the billions of dollars instead of the small numbers normally seen in dentistry.
Treatment of periodontitis is likely to move in the direction of new endpoints that influence the medical events, and so people will want that to be noted, to be measured, and to be documented, and medical insurance will have a real incentive for promoting and encouraging what is a relatively low-cost approach to reducing cardiovascular events and other illnesses.
As for the patients of today and in the future, they are certain to benefit from strides in inflammation control and regeneration, and their dentist will continue to strive to meet their expectations as they evolve, too.
About the Authors
Donald S. Clem III, DDS
Private Practice
Fullerton, California
Kenneth S. Kornman, DDS, PhD
Chief Scientific Officer
Interleukin Genetics
Adjunct Faculty
Harvard School of Dental Medicine and the University of Michigan School of Dentistry
References
1. Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914-920.
2. Jimenez MC, Sanders AE, Mauriello SM, et al. Prevalence of periodontitis according to Hispanic or Latino background among study participants of the Hispanic Community Health Study/Study of Latinos. J Am Dent Assoc. 2014;145:805-816.
3. Centers for Disease Control and Prevention. Fast Stats: Death and Mortality. Available at: http://www.cdc.gov/nchs/fastats/deaths.htm. Accessed: August 8, 2015.
4. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217-226.
5. Sokolosky ML, Wargovich MJ. Homeostatic imbalance and colon cancer: the dynamic epigenetic interplay of inflammation, environmental toxins, and chemopreventive plant compounds. Front Oncol. 2012;2:57.
6. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314-321.
7. Genco RJ, Schifferle RE, Dunford RG, et al. Screening for diabetes mellitus in dental practices: a field trial. J Am Dent Assoc. 2014;145:57-64.
8. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59-94.
9. Daubert DM, Weinstein BF, Bordin S, et al. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Perio. 2015;86:337-347.
10. Gomez-Moreno G, Aguilar-Salvatierra A, Rubio Roldan J, et al. Peri-implant evaluation in type 2 diabetes mellitus patients: a 3-year study. Clin Oral Implants Res. 2015;26:1031-1035.
11. Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Perio. 1996;67:1085-1093.
12. Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Perio. 2013;84:S135-S152.
13. Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306-311.
14. Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27-32.
15. Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Perio. 2013;84:S153-S169.
16. Munenaga Y, Hiroshima Study Group, Yamashina T, et al. Improvement of glycated hemoglobin in Japanese subjects with type 2 diabetes by resolution of periodontal inflammation using adjunct topical antibiotics: results from the Hiroshima Study. Diabetes Res Clin Pract. 2013;100:53-60.
17. Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310:2523-2532.
18. Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep. 2013;15:295.
19. Dietrich T, Sharma P, Walter C, et al. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Perio. 2013;84:S70-S84.
20. Hood L. Systems biology and p4 medicine: past, present, and future. Rambam Maimonides Med J. 2013;4:e0012.
21. Giannobile WV, McClain PK. Enhancing periodontal health through regenerative approaches. J Periodontol. 2015;86(2 Suppl):1-3.
22. Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597-605.
23. Beck J, Garcia R, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123-1127.
24. Jackson JM, Kircik LH, Lerenz DJ. Efficacy of extended-release 45 mg oral minocycline and extended-release 45 mg oral minocycline plus 15% azelaic acid in the treatment of acne rosacea. J Drugs Dermat. 2013;12(3):292-298.
25. Jeffcoat MK, Jeffcoat RL, Gladowski PA, et al. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med. 2014;47(2):166-174.