The ADAA has an obligation to disseminate knowledge in the field of dentistry. Sponsorship of a continuing education program by the ADAA does not necessarily imply endorsement of a particular philosophy, product, or technique.
Enamel is the hardest substance in the body, and it protects the crowns of the teeth. However, it is susceptible to demineralization from acids. Acids are produced when certain bacteria colonize the tooth surface and metabolize carbohydrates. If this process continues it may eventually lead to the development of carious lesions in the enamel and dentin. Many foods and beverages contain acids that also can lead to demineralization of the enamel.
Soda pop has emerged as one of the most significant dietary sources of acid capable of producing demineralization of the enamel. Many brands of soda pop also contain sugars that are fermented by bacteria that produce acid by-products. It also appears that soda pop contains other ingredients that produce demineralization independent of its acid content or fermentable sugars.1 The role of soda pop in the demineralization of enamel and its consequences should not be underestimated.
Soda Pop Consumption
The consumption of soda pop in the United States has increased to alarming proportions. This increase in consumption crosses all demographic boundaries, with many Americans drinking more soda pop and drinking it more frequently. This has created a public health crisis, which has been recognized by a number of professional associations. Soda pop consumption has increasingly become a factor in oral disease. Clinically, demineralization occurs, with erosion of tooth surfaces and caries being evident; the most severe effects are seen in people who drink several servings a day.
Recently the American Academy of Pediatrics published a position paper to inform health care professionals, school personnel, and parents about the significant dangers posed by the ever-increasing amounts of soda pop consumed by children and teenagers.2 Between 56 percent and 85 percent of school-age children consume at least one serving of soda pop each day, and often the amount of soda pop consumed daily is much larger. At least 20 percent of school-age children consume a minimum of four soda pop servings every day.3 Some of these trends are summarized in .
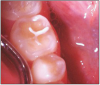
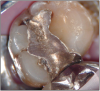
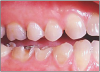
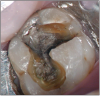
The potential ravages of soda pop caries in teenagers should not be underestimated. Some teenagers drink as many as 12 cans of soda pop a day. In one well-documented case, a teenager who grew up drinking fluoridated water and brushing twice daily with a fluoride-containing toothpaste developed caries in every one of his erupted teeth, necessitating two extractions and many restorations. Diet analysis revealed that he consumed 6-12 cans of soda pop daily.4 Some of the ravages of this condition are visible in and . Other case reports have demonstrated similar findings among other adolescents in whom chronic, high soda pop consumption was linked with widespread demineralization of enamel and extensive caries in pits and fissures and in the interproximal areas.5
Soda Pop in Schools
One major development in this problem has been the access to soda pop in schools. Many schools throughout the country have easy access to commercial soda pop vending machines in the schools, and students have free and easy access to purchase soda pop at will.
Some commercial soda pop vendors provide deep discounts to the schools to allow them to place their vending machines on school premises. In times of budgetary constraint these offers may be difficult to resist, and some schools have signed lucrative contracts with vendors. Student governments may also favor the placement of soda pop dispensing machines in schools.
This has become a controversial issue in some schools. Some parent-teacher organizations have sought to have soda pop vending machines removed from school premises, which may lead to hotly contested conflicts at meetings at various levels in the school districts. Sometimes the vending machines are removed, and sometimes the machine contents are replaced with diet sodas, 100% fruit juices, and water.
Soda Pop in the Marketplace
Soda pop has become a firmly entrenched staple of the American diet and as American as apple pie. The commercial soda pop manufacturers have invested a fortune in advertising and have created one of the most successful marketing campaigns in American history. Soda pop has become an integral part of American culture.
In the 1950s the typical soda pop serving size was 6.5 oz., by the 1960s this increased to 12 oz., and in the 1990s the typical serving size ballooned up to 20 oz. It is clear that not only are we drinking more soda pop but also that we are buying it in ever-increasing amounts. In the U.S., soda is packaged in 8, 12, 14, 16, 20, and 24-ounce cans and bottles as well as 1, 2, and 3-liter bottles. This trend is also reflected in fast food outlets, which have been steadily increasing the volume of soda pop in each of their beverage serving sizes. Soda size selections in some fast food restaurants can range from a small 16 oz., medium 21 oz., large 32 oz., and even a super-sized 44 oz. serving or larger.
A Hidden Danger (Accompanying or Associated)
One of the concomitant problems with the increase in soda pop consumption is that it leads people to drink less milk, which indirectly leads to a higher incidence of demineralization and caries. Milk contains calcium lactate, which stimulates remineralization of enamel.6 The regular consumption of adequate quantities of milk bathes the teeth in calcium and calcium lactate and promotes remineralization7 to combat the demineralization and erosion caused by soda pop. Thus on the one hand the means for combating enamel erosion is being compromised because people are drinking less milk and on the other hand the increased consumption of soda pop contributes to the more rapid and extensive demineralization of the enamel.8 Associations have also been found recently between high consumption of soda pop (at the expense of healthier drinks, such as milk, which contains vitamins and minerals) and osteoporosis.
Bacteria Produce Acids That Demineralize Enamel
Dental caries is an infectious, chronic, multifactorial disease.9 The disease process is initiated when bacteria are passed from the parent to the infant or toddler. These bacteria later colonize the outer surface of the enamel, form dental plaque and begin metabolizing carbohydrates, such as the sugars sucrose and fructose, which causes a lowering of the pH of saliva and a consequent demineralization of the enamel. When the pH drops below 5.5 for long or repetitive periods, there is a significant chance that this demineralization will lead to the development of carious lesions in the enamel. Streptococcus mutans is the most significant of the bacteria involved in the development of dental caries. Lactobacillus and Actinomyces viscosus colonize later and are also important in generating acid by-products.
Enamel experiences continual cycles of demineralization and remineralization and is a dynamic process that can proceed in either direction. Factors on both sides of this equation may change, shifting the reaction in one direction or the other. For many people and in many cases, increasing the sugar content of their diet can increase demineralization and increase the chance that this may eventually lead to the development of caries.
Soda pop is most commonly sweetened by adding sucrose or high-fructose corn syrup, which is the equivalent of 10-12 teaspoons of sugar in the typical 12-oz. can of naturally sweetened soda pop. These sugars fuel the metabolism of bacteria that produce the acids which demineralize enamel. For many people, soda pop is the single biggest source of sugar in their diet. The greater the exposure to these sugars, the more acid produced by the bacteria and the greater the chance of demineralization.
Soda Pop and Acid
In the past, the focus of the deleterious effects of soda pop has been on its sugar content and its role in sustaining bacterial growth and acid by-products. However, it is clear now that there are two significant threats posed by soda pop. The sugar content certainly does fuel the bacteria that produce acidic by-products, which does have a significant effect on the demineralization of enamel and development of caries. Soda pop also exerts a profound, deleterious effect by bathing the teeth in acid that also is capable of producing demineralization.
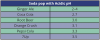
There is no question that enamel can be demineralized by exposure to soda pop.10 Depending on the kind and brand, soda pop may contain carbonic, phosphoric, malic, citric, and tartaric acids and have an acidic pH.11 Some soda pops that have an acidic pH are listed in .12 Repeated exposure to these acids produces demineralization and erosion of the enamel. Demineralization of enamel is inversely related to the pH of the soda pop. The more acidic the soda pop (i.e., the lower its pH), the more rapid and profound the demineralization of the enamel.13
Brands of soda pop that contain artificial sweeteners still pose a significant threat because of their acid content. While they may not contain sucrose or fructose or other fermentable carbohydrates, their acidic content will contribute to the demineralization of enamel. Their threat may not be as great, but they are still capable of producing demineralization. In any case these brands of soda pop only account for 14 percent of the market share.14
Long-term consumption of soda pop has a significant, cumulative effect on the demineralization of enamel.15 The older a person is and the longer that person has been drinking soda pop, the more likely that person will have a higher than expected decayed, missing, and filled surfaces (DMFS) score. In people 25 or older there is a statistically significant association between long-term soda pop consumption and higher than expected DMFS.16 As more people are living longer, the teeth of those long-term soda pop drinkers will be experiencing more chemical erosion of the enamel, with consequent demineralization and dissolution of tooth structure and development of caries.
How Saliva Buffers Acids
One of the body's most effective means for protecting the enamel of the teeth against acid is saliva. Saliva contains many components such as calcium ions, phosphorus, proteins, enzymes, and bicarbonates. One of its most important functions is to bathe the teeth in a supersaturated solution of calcium and phosphorus so that the enamel of the teeth is constantly exposed, to replace any loss of tooth structure due to demineralization. A second function of saliva is to buffer the pH of saliva to prevent the oral environment becoming too acidic.17 Normal salivary pH is about 6.3. When the pH of saliva drops below 5.5, demineralization usually follows.
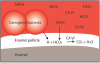
The mechanism for the buffering effect of saliva involves the activity of the bicarbonate ions. As the acid content of saliva increases, the concentration of hydrogen ions increases, which lowers the pH. The enzyme carbonic anhydrase found in saliva catalyzes the reaction between the free hydrogen ions from the acid and the bicarbonate ions.18 The end products of this reaction are water and carbon dioxide gas, which is released from the oral cavity, as depicted in . As more free hydrogen ions combine with bicarbonate ions, the pH begins to rise and the saliva begins to return to normal pH levels.19
The buffering capacity of saliva varies from person to person. Patients whose saliva has a depressed buffering capacity are more susceptible to erosion from acid.20 Salivary flow also determines the capacity of saliva to buffer against acid. The greater the salivary flow, the more bicarbonate ions are available for combining with free hydrogen ions. When acid is introduced into the oral cavity, salivary flow is stimulated and increases within minutes.
Normal salivary flow rates are generally between 0.1 and 0.6 mL per minute.21 Salivary flow of less than 0.1 mL per minute is considered low.22
The chemical reaction between the hydrogen ions released from acids and the bicarbonate ions in saliva protects the enamel from demineralization. Without this protective buffering capacity of saliva, enamel would be demineralized and lost. However, this buffering capacity of saliva is limited and can be overwhelmed by frequent or long-term exposure to acids.
The Dangers of Softened Enamel
When enamel is softened by exposure to soda pop, it is at increased danger of being worn away or abraded. This may result in a synergism with other causes of tooth structure loss, such as from vigorous tooth brushing with a hard-bristle toothbrush or from bruxism.
Recognizing the Signs of Soda Pop Erosion
Patients with soda pop erosion present with certain changes in the morphology and surface characteristics of their teeth. Smooth surface enamel may develop broad, shiny concavities. The teeth may even have a glazed appearance.23 Mandibular premolars and molars commonly develop wide concavities on their buccal surfaces in the cervical third. These concavities may terminate at the free gingival margin, producing a characteristic enamel cuff at the free gingival margin, or they may extend on to the root surface if the roots are exposed. The occlusal surfaces of premolars and molars may be punctuated by deep, shiny concavities that may extend down into the dentin. The occlusal surfaces that have been partially restored may demonstrate loss of enamel around the occlusal aspect of the restoration so that it appears to rise above the existing occlusal surface. These features can be seen in and . The maxillary central incisors may appear thinner with an increase in incisal translucency. The surface will appear polished and smooth, and distinctive surface characteristics will be missing. Erosion of the occlusal surface of permanent first molars with sealants may result in the sealants appearing to rise above the occlusal surface. Erosion from soda pop in the primary dentition will show as a loss of surface definition and detail. The enamel and dentin layers are much thinner than in the permanent dentition and there is an increased chance of erosion leading to pulp exposure.24 ()
Recognizing Patients at High Risk
Diminished Salivary Flow and Xerostomia
Low salivary flow means less saliva is available to rinse the soda pop and acid by-products off the teeth and fewer bicarbonate ions are present to buffer the acids in soda pop and the acids produced by the fermentation of sugars. Some of the more common, visible signs of low salivary flow include dryness of the lips and buccal mucosa. The dorsum of the tongue may also appear dry and cracked.
The major salivary glands should be palpated and milked. Gentle massaging of the parotid gland, or simply swabbing the duct opening with a gauze square, should result in the free flow of saliva from Stensen's duct. The submandibular gland should also be gently massaged, and saliva should flow freely from Wharton's duct. The saliva should be clear and flow freely. Palpating these glands during the oral exam will also help to detect salivary stones which can lead to blocked ducts and a reduction in saliva flow.
Low salivary flow can be caused by medical conditions that affect the function of the salivary glands and by certain drug therapies. Some of the more common medical conditions that cause diminished salivary flow include radiation therapy to the head and neck, poorly controlled diabetes, and Sjogren's (SHOW-grins) syndrome, a disorder of the immune system identified by its two most common symptoms-dry eyes and a dry mouth. The condition often accompanies other immune system disorders, such as rheumatoid arthritis and lupus. Some of the more common drugs that produce diminished salivary flow include alpha blockers and antihistamines.
Some patients with xerostomia or diminished salivary flow may suck on sugary candies or sip soda all day long to combat the sensation of dryness in their mouths. This continuous or repeated exposure to soda pop in the absence of the protective benefits of saliva can be devastating.
Destructive Habits
Some patients have destructive habits involving the consumption of soda pop. For example, some patients derive pleasure from holding soda pop in their mouth and allowing it to bathe certain teeth. Some may actually swish the soda pop around and around for several minutes before swallowing. The carbonation and effervescence of the soda pop produce a pleasing sensation which can lead to excessive erosion of these exposed teeth in affected areas.25
Orthodontics
Demineralization and caries have been traditional dangers with cemented brackets in fixed orthodontics. Patients undergoing orthodontic therapy must practice meticulous oral hygiene in order to protect their teeth. Increased soda pop consumption poses a significant threat to the development of caries around fixed orthodontic appliances. In one case report, a teenager who consumed 2-4 liters of cola soda pop presented with significant demineralization around and under fixed cemented brackets. In some cases the demineralization led to a loss of 0.5 millimeters of enamel.26 Patients with orthodontic bands have a significantly higher prevalence of Streptococcus mutans.27
Meticulous oral hygiene and home care are essential to protect teeth with bands or bonded brackets. Consideration may be given to the added protection of placing sealant around the margins of the bands or brackets to enhance the sealing effect of the cement.28
Another simple procedure for reducing the demineralizing effects of soda pop erosion around orthodontic brackets is to place fluoride varnishes around the brackets.29 This procedure involves the teeth with brackets being isolated and dried and placing fluoride around the edges of the brackets and the adjacent tooth surfaces. This therapy has proved to be an effective means of preventing demineralization30 and the formation of caries.31
Role of the Dental Team
One of the most effective techniques for identifying patients at high risk of soda pop-related erosion and caries is to assess how often and how much soda pop is consumed. Patients should be asked how often and how much soda pop they drink. Some patients only drink soda pop at meals, some in between meals as well and some all day long. Patients may also be able to describe how much soda pop they drink in a day or week. Patients should be asked about the kinds of soda pop they drink. Some soda pops are more acidic than others and some contain more sugar, and some soda pops contain artificial sweeteners and so do not pose a threat from the perspective of bacteria metabolizing sugars.
Home care and oral hygiene should be assessed. The dental professional should ask the patient how many times he or she brushes and which brand of toothpaste the patient uses. The patient should also be asked if he or she uses a fluoride mouth rinse. Many patients will also know whether their water source is fluoridated.
Diet Counseling
One of the most important things a dental team can do is to provide patients with diet counseling. Children and adolescents should be counseled to avoid consuming large amounts of soda pop.32 They should be counseled to drink more alternative beverages that contain less sugar and acid such as water, milk and 100 percent fruit juice. Their parents should also be informed and counseled and should understand how to stock their refrigerators and to replace fruit drinks with high sugar content with 100 percent fruit juices and encourage their children to drink these instead. Parents must become informed and involved and must be proactive in encouraging their children to develop more healthful habits.
For some patients, the consumption of soda pop is addictive, and this may occur at an early age and continue through life. There are no safe beverages (i.e., non-acidic, non-cariogenic) that can be used to compete with soda pop. The soda pop flavors and carbonation are supreme; therefore, when soda pop becomes an addiction, diet counseling should focus on coping behaviors.
Adults should be counseled on the dangers of soda pop consumption and should be encouraged to drink more healthful beverages. Destructive habits such as sipping soda pop all day long at work should be identified and discouraged. This is also key for a xerostomic patient in the habit of relieving his or her dry mouth by sipping soda pop all day. The patient can also be counseled to rinse with water after drinking soda pop to evacuate the oral cavity of any remaining vestiges of soda pop, which might prolong exposure of the enamel.33 Rinsing with water-especially fluoridated water-after eating and after drinking soda pop also reduces the Streptococcus mutans load in the oral cavity, thus decreasing caries activity.34 Patients should be encouraged to do this especially at night if they wake up and drink soda or have a snack. It might be unrealistic to expect them to forego their soda or snack or to brush their teeth again, but it is realistic to encourage them to rinse with water after they finish drinking or eating their midnight snack. Patients should be advised to drink tap water if they live in a fluoridated area, and if they drink bottled water they should be advised to check that the brand of bottled water they are drinking contains fluoride as some do and others do not.
Patients with GERD are especially at risk because of the volumes of gastric acids regurgitated and the potential for acid erosion of their teeth. Some physicians recommend that these patients consume five small meals per day instead of the usual three. In situations like this, rinsing with fluoridated water after consuming a small meal will help clear the mouth and protect the teeth. These patients should be counseled to brush after as many meals as can be accommodated and to rinse with fluoridated water for those instances when they do not brush.
Therapies to Increase Fluoride
Increasing the patient's exposure to fluorides is one means of combating the demineralizing effect of soda pop. The topical effects of fluoride exposure on erupted teeth have been well documented.35 When patients present for scheduled oral prophylaxis, fluoride should be applied in relatively high doses. The patient should be counseled to use fluoride mouth rinses and fluoride toothpaste as part of regular home care. Repeated exposure to fluoride within safe limits stimulates remineralization and prevents further demineralization and erosion.
Professionally Applied Fluoride
When the patient presents for an oral prophylaxis, fluoride should be applied. The controlled application of relatively high doses of fluoride on a regular basis is one significant reason for patients to present for professional oral prophylaxis. The traditional means of application involves use of a fluoride gel in an applicator tray. Fluoride can also be applied as a foam substance delivered in a tray that decreases the chance of the patient swallowing excess fluoride. Gels and foams can be either acidulated phosphate fluoride or neutral sodium fluoride. Using neutral sodium fluoride is advisable in patients with ceramic restorations to avoid potential etching of the restorations by acidulated formulations. A number of gel and foam products are listed in .
Fluoride Varnishes
One of the most significant improvements in the delivery of fluoride to teeth involves the use of 5% sodium fluoride varnishes that contain high concentrations of fluoride (22,600 ppm). Dental erosion can result in dentinal hypersensitivity once the enamel or cementum has been eroded away. Fluoride varnish is proven to relieve hypersensitivity and does so by the action of the globules blocking the dentinal tubules, while at the same time, the fluoride varnish forms globular calcium fluoride on the surface of the teeth. This serves as a reservoir and releases fluoride ions in response to depressions in the pH of the oral cavity.36 This increase in fluoride availability promotes remineralization and decreases demineralization.
The fluoride varnish is applied to tooth surfaces and allowed to dry and harden. For best results, the teeth are dried and isolated, and varnish is applied with a brush applicator. The procedure is fast, simple and effective.
Fluoride varnish reduces demineralization on the surfaces where it has been directly applied (i.e., buccal, lingual and occlusal) as well on adjacent surfaces where application is not possible (i.e., the interproximal surfaces).37-39 Fluoride varnishes have been shown to reduce the softening of enamel under acid challenge from soda pop40 and to reduce soda pop chemical erosion41 as well as reducing erosive wear.42
One effective regimen for applying fluoride varnish is at six month intervals.43 Fluoride varnishes have demonstrated release of fluoride over time, up to six months following a single application.44 This regimen can easily be accommodated into the customary scheduling of recall prophylaxis, which is generally every six months for patients who quickly develop stain and calculus. For high-risk patients, multiple applications of fluoride varnish over a short time span will result in higher concentrations of fluoride being released, affording more protection from hypersensitivity and acid challenge.45 Alternatively, applications could be performed four times per year instead of the typical twice per year for high-risk patients.
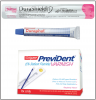
In many dental practices around the world, fluoride varnish is replacing fluoride gel or foam for the professional application of high-concentration fluoride because of the proven efficacy of fluoride varnish46 and the ease of application. The procedure does not involve the insertion of custom trays or entail any of the potential problems of fluoride gels and foams such as ingestion of fluoride, the resulting nausea, or the discomfort of having trays in the mouth for up to four minutes. Brush or swab application is quick and easier for patients than trays, especially in children and gagging patients. Available fluoride varnishes include PreviDent® Varnish and Duraphat® (Colgate Oral Pharmaceuticals), Duraflor varnish (Pharmascience), EnamelPro® (Premier), and Flor-Opal Varnish (Ultradent). ()
Fluoride Mouth Rinses
Rinsing with a mouthwash containing fluoride reduces the incidence of caries and stimulates remineralization. In fluoride-deficient areas rinsing once a week with 0.2% sodium fluoride or daily with 0.05% sodium fluoride both significantly reduce the incidence of caries in children.47 In one school-based preventive dental program, rinsing once a week with 0.2% sodium fluoride resulted in a reduction of 85 percent in the incidence of caries on proximal surfaces.48
There are numerous mouth rinses with 0.05% sodium fluoride that can be purchased over the counter that can be recommended to the patient. Patients should be advised to use one once daily as a regular part of their home care oral hygiene regimen. For patients with higher risk, a prescription for a mouth rinse with 0.2% sodium fluoride can be written with instructions for the patient to rinse once a week.
Fluoride Toothpastes and Gels
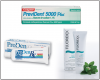
Patients should brush with a fluoride-containing toothpaste. For patients at higher risk, a prescription can be given for toothpaste with higher fluoride content containing 1.1% sodium fluoride and 5000 ppm fluoride. A number of these are available including PreviDent 5000 Plus® (Colgate), ProDenRx (ProDentec) and Fluoridex® (Discus Dental). ()
One 1.1% sodium fluoride formulation, PreviDent® 5000 Booster (Colgate Oral Pharmaceuticals) has been created to increase the speed of fluoride dispersion to the teeth.49 If the patient is experiencing hypersensitivity as well, a formulation is available containing both 1.1% sodium fluoride in combination with the FDA-mandated level of potassium nitrate for hypersensitivity relief (PreviDent® 5000 Sensitive). ()
After brushing with a fluoride toothpaste or higher fluoride level dentifrice, adult patients should be encouraged to rinse with a small amount of water50 or to just spit out the excess.51 This will result in a much higher concentration of fluoride remaining on the teeth for a longer period of time, which will afford significantly more protection.52 High-risk patients should be supplied with custom trays and high-fluoride-content gels or pastes for maximum fluoride protection. These patients should be encouraged to use these daily as directed.
Sealants
While sealants are effective against preventing Class I pit and fissure caries, they may not be a long-term effective barrier to the demineralizing effects of soda pop. In one in vitro study, teeth were etched and sealed and then immersed in nine different dark cola soda pops. All teeth showed complete or incomplete loss of sealant and significant demineralization.53
Keeping the Public Informed
Numerous articles have appeared in newspapers and magazines informing the public of the dangers of soda pop to teeth and to general health.54 Some articles focus on the dangers of consuming too much soda pop and recommend alternative beverages.55 Some articles describe in detail how soda pop produces demineralization and caries.56 Some articles describe new patterns of caries that are becoming more common with increased and chronic consumption of soda pop.57
A great deal of information about the dangers of soda pop can be accessed on the Internet through many websites. Several websites (
http://www.healthy-environments.com/Soda-Pop-Dangers.asp) offer information regarding the harmful effects of drinking too much soda pop, leading to many health problems including tooth decay.
58 WebMD also has an excellent article on soda pop and caries. (
http://www.webmd.com/oral-health/news/20010712/too-much-soda-taking-its-toll-on-kids-teeth).
59Summary
The consumption of soda pop in the United States continues to increase in alarming proportions with consequent drastic effects on the dentition of many people. Patients should be asked about how much soda pop they ingest. Parents should be counseled on the effects of soda pop demineralization and begin to limit the amounts given to children at home and in schools. The dental team has the expertise and training to intervene with diet counseling, home care instruction and professionally applied fluoride to decrease the potential ravages of soda pop.
Glossary
Analysis - the examination of something in detail in order to understand it better or draw conclusions from it.
Bicarbonates - same as hydrogen carbonate.
Carabonic Acid Salt - a salt of carbonic acid in which one hydrogen atom has been replaced, usually by a metal.
Carbonic Anhydrose - containing carbon.
Calcium Ions - a soft, silvery-white element that is an alkaline earth metal constituting about three percent of the earth's crust. It is essential to the formation of bones and teeth.
Citric - relating to citrus fruit.
Compromised - exposed somebody or something to danger.
Concomitant - happening at the same time as something else.
Cumulative - becoming larger, stronger. Statistics describes an error that increases as more measurements are taken.
Demineralization - to remove minerals or mineral salts from something such as bone, teeth or a liquid.
Enzyme - any complex chemical produced by living cells that is a biochemical catalyst.
Fluoride Ions - a chemical compound consisting of fluorine and another element or group.
GERD- gastroesophageal reflux disease; a condition when stomach contents leak backward into the esophagus.
Hydrogen Ions - a positively charged ion of hydrogen that is formed by the removal of an electron from a hydrogen atom and is present in solutions of acids in water. The degree to which a compound produces hydrogen ions in solution is measured on the pH scale, 1 being highly acidic, 7 being neutral, and 14 being highly alkaline.
Malic - relating to or derived from malic acid; found in unripe fruits and is used as an active ingredient in tart, sour foods.
Metabolizing - to subject something to metabolism, or undergo metabolism.
Morphology - the study of the form and structure of organisms.
Multi Factorial - many, multiple, more than one or two.
pH - a measure of acidity or alkalinity in which the pH of pure water is 7, with lower numbers indicating acidity and higher numbers indicating alkalinity.
Phosphoric - containing phosphorous with a valence state higher than that of the phosphorus ion or radical in an analogous phosphorous compound
Remineralize - replacement of depleted mineral content of teeth.
Stimulates - to encourage something such as an activity or a process so that it will begin to increase, or develop; to cause somebody to become interested about something.
References
1. Von Fraunhofer J, Rogers M. Dissolution of dental enamel in soft drinks. Gen Dent. 2004;52:308-12.
2. American Academy of Pediatrics Committee on School Health. Pediatrics. 2004;113:152-4.
3. Gleason P, Suitor C. Children's diets in the mid 1990s: Dietary intake and its relationship with school meal participation. Alexandria, VA: US Department of Agriculture, Food and Nutrition Service, Office of Analysis, Nutrition and Evaluation; 2001.
4. Brimacombe C. The effect of extensive consumption of soda pop on the permanent dentition: A case report. NW Dent. 2001;80:23-5.
5. Majewski R. Dental caries in adolescents associated with caffeinated carbonated beverages. Pediat Dent. 2001;23:198-203.
6. Beiraghi S, et al. Effect of calcium lactate in erosion and S. mutans in rats when added to Coca-Cola. Pediat Dent. 1989;11:312-5.
7. Gedalia I, et al. Enamel softening with Coca-Cola and rehardening with milk or saliva. Am J Dent. 1991;4:120-2.
8. Grenby T, Andrews A, Mistry M, Williams R. Dental caries-protective agents in milk and milk products: investigations in vitro. J Dent. 2001;29:83-92.
9. Adair S, et al. Recommendations for using fluoride to prevent and control dental caries in the United States. Mortality and Morbidity Weekly Report. 2001;50:1-42.
10. Gedalia I, et al. Tooth enamel softening with a cola type drink and rehardening with hard cheese or stimulated saliva in situ. J Oral Rehabil. 1991;18:501-6.
11. Roos E, Donly K. In vivo dental plaque pH variation with regular and diet soft drinks. Pediat Dent. 2002;24:350-3.
12. Clark D, et al. The influence of frequent ingestion of acids in the diet on treatment for dentin sensitivity. J Can Dent Assoc. 1990;1101-3.
13. Larsen M, Nyvard B. Enamel erosion by some soft drinks and orange juices relative to their buffering effect and contents of calcium phosphate. Caries Res. 1999;33:81-7.
14. Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: Nutritional consequences. J Am Dietet Assoc. 1999;99:436-44.
15. Heller K, Burt B, Eklund S. Sugared soda consumption and dental caries in the United States. J Dent Res. 2001;80:1949-1953.
16. US Department of Health and Human Services (USDHHS). National Center for Health Statistics (1997). National Health and Nutrition Examination Survey III, 1988-1994, Series 11, No. 1A, Hyattsville, MD; Centers for Disease Control and Prevention.
17. Tenovo J, ed. Human Saliva: Clinical Chemistry and Microbiology, vol. 1. Boca Raton: CRC Press; 1989:44-59.
18. Kivela J, et al. Salivary carbonic anhydrase isoenzyme VI. J Physiol. 1999;520:315-20.
19. Perkins S, Wetmore M. Acid-induced erosion of teeth. Dent Today. 2001;20:82-7.
20. Gudmundsson K, et al. Tooth erosion, gastroesophageal reflux and salivary buffer capacity. Oral Surg Oral Med Oral Pathol. 1995;79:185-9.
21. Janvinen V, Rytomaa I, Heinonen O. Risk factors in dental erosion. J Dent Res. 1991;70:942-7.
22. Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71:1363-9.
23. Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Research 2004;38:34-44.
24. Milosevic A. Tooth wear: An aetiological and diagnostic problem. Eur J Pros Restor Dent. 1993;1:173-178.
25. Gandara B, Truelove. Diagnosis and management of dental erosion. J Contemp Dent Pract. 1999;1:1-15.
26. Prietsch J, de Souza M, de Souza G. Case report: unusual dental erosion caused by a cola drink. J Clin Orthod. 2002;36:549-552.
27. Corbett J, et al. Comparison of Streptococcus mutans concentrations in non-banded and banded orthodontic patients. J Dent Res. 1981;60:1936-42.
28. Liebenberg W. Quintessence International. 1994;25:303-312.
29. Schmit JL, et al. Effect of fluoride varnish on demineralization adjacent to brackets bonded with RMGI cement. Am J Orthod Dentofacial Orthodop. 2002;122:125-134.
30. Gontijo L, et al. Dental enamel around fixed orthodontic appliances after fluoride varnish application. Braz Dent J. 2007;18:49-53.
31. Stecksen-Blicks C, et al. Caries-preventiveness of a fluoride varnish; a randomized controlled trial in adolescents with fixed orthodontic appliances. Caries Res. 2007;41:455-459.
32. Krebs-Smith S. Choose beverages and foods to moderate your intake of sugars: Measurement requires quantification. J Nutrition. 2001;131:5275-5355.
33. Larsen M. Erosive drinks and pH of the tongue saliva. Int Assoc Dent Res. 2004, Abstract 2050.
34. Shrestha B, et al. Mouthrinsing effects on oral mucosal clearance in children. J Dent Res. 2002;81:Abstract 4053.
35. Limeback H. A re-examination of the pre-eruptive and post- eruptive mechanism of the anti-caries effects of fluoride: Is there any anti-caries benefit from swallowing fluoride? Comm Dent Oral Epidemiol. 1999;27:62-71.
36. Shen C, Autio-Gold J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. J Am Dent Assoc. 2002;133:176-82.
37. Petersson LG, et al. The efficiency of semiannual fluoride varnish applications: a two year clinical study in preschool children. J Pub Health Dent. 1998;58:57-60.
38. Petersson LG, et al. Effect of semi-annual application of chlorhexidine/fluoride varnish mixture on approximal caries incidence in schoolchildren. A three year radiographic study. Eur J Oral Sci. 1998;106:623-7.
39. Stahl J, Zandona A. Rationale and protocol for the treatment of non-cavitated smooth surface carious lesions. Gen Dent. 2007;55:105-1.
40. Magalhaes AC, et al. Effect of an experimental 4% titanium tetrafluoride varnish on dental erosion by a soft drink. J Dent. 2007;35:858-61.
41. Sorvari R, et al. Effect of fluoride varnish and solution on enamel erosion in vitro. Caries Res. 1994;28:227-32.
42. Viera A, et al. Inhibition of erosive wear by fluoride varnish. Caries Res. 2007;41:61-7.
43. Kallestal C, Fieldahl A. A four year cohort study of caries and its risk factors in adolescents with high and low risk at baseline. Swed Dent J. 2007;31:11-25.
44. Castillo JL, et al. Evaluation of fluoride release from commercially available fluoride varnishes. J Am Dent Assoc. 2001;132:1389-92.
45. Castillo JL, Milgrom P. Fluoride release from varnishes in two in vivo protocols. J Am Dent Assoc. 2004;135:1696-7.
46. Kallestal C, Fjelddahl A. A four-year cohort study of caries and its risk factors in adolescents with high and low risk at baseline. Swed Dent J. 2007;31:11-25.
47. Heifetz S, et al. A comparison of the anticaries effectiveness of daily and weekly rinsing with sodium fluoride solutions: Final results after three years. Pediat Dent. 1983;4:300-3.
48. Leske G, et al. Post-treatment benefits in a school-based fluoride mouth rinsing program. Clin Prev Dent. 1986;8:19-23.
49. Joziak, MT, et al. Comparison of enamel fluoride uptake and fluoride release from liquid and paste dentifrice. J Dent Res. 2003;82(Sp. Issue). Abstract 1355.
50. Duckworth RM, et al. Effect of mouthrinsing after toothbrushing. Caries Res. 1991;25:287-91.
51. Sjogren K, et al. Effect of water rinsing after toothbrushing. Caries Res. 1994;28:455-9.
52. Sjogren K, Melin NH. The influence of fluoride retention after toothbrushing. Gerodontol. 2001;18:15-20.
53. Steffen J. The effects of soft drinks on etched and sealed enamel. Angle Orthod. 1996;66:449-56.
54. MacDonald Sue. Tooth decay and the soda factor. The Cincinnati Enquirer. April 7, 1999.
55. La Duca D. Are we drinking too much soda pop? Colorado State University Food Stamp Nutrition Education Program. August 21, 2001.
56. Mendenhall D. The Pittsburgh Post Gazette. September 4, 2001.
57. Dental association blames children's tooth decay on too much soda pop. The Detroit News. April 1, 2000.
59. Davis J. Too much soda taking its toll on kids' teeth.
http://www.webmd.com/content/article/33/1728_83581.
About the Author
Gary J. Kaplowitz DDS, MA, MEd
Dr. Kaplowitz retired from the military after serving as Chief Dental Officer of the US Coast Guard. He is a graduate of the New York University College of Dentistry and is a Diplomate of the American Board of General Dentistry. He has published widely in peer-reviewed journals on dental materials and clinical techniques. He is editor-in-chief of
www.Osseonews.com, an implant website, and is in private practice in York, Pennsylvania. He maintains a dental consulting practice in Baltimore for product development and research. He is a clinical associate on staff at the University of Pennsylvania School of Dentistry in the Department of Oral Medicine.