You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Disclosures: The author had no disclosures to report.
Several factors involved in the success of an implant-supported restoration include the biocompatibility of the implant fixture and the type of surrounding bone, the design of the occlusion, and retention of the restoration.1 The connection between the restoration and implant is accomplished either by cementation, screw retention, or a combination of both.2 Cost, accessibility, esthetics, occlusion, and ease of fabrication are factors to consider when determining the type of retention to use.3 The cement-retained implant-supported restoration is easier to fabricate than a screw-retained restoration because conventional crown-and-bridge laboratory techniques are employed.4 The screw-retained implant-supported restoration involves more complicated, time-consuming laboratory procedures and, therefore, is more costly.5 The success rates between the two types of retention have been evaluated in several studies.6-10 Most of these studies have found the screw-retained restoration has had more complications than their cement-retained counterparts, but they are usually minimal.3
Problems With Cement-Retained Implant-Supported Crowns
A cement-induced peri-implantitis associated with cement-retained restorations has been reported.11 This so-called cementitis, or peri-implantitis, is accompanied by an unfavorable loss of marginal bone and represents a common problem associated with cement-retained implant supported restorations.12 Pauletto et al13 reported, within weeks of the placement of the cemented implant-supported restoration, clinical signs may be present. The incomplete removal of cement can lead to gingival inflammation, swelling of the tissue, soreness, bleeding, or exudation on probing, resulting in resorption of the adjacent crestal bone.14 The gingival response, though, has always been the most favorable when no cement was used.3
A number of methods have been purposed for preventing the presence of excess cement at the restoration/abutment interface. These techniques include the use of plastic scalers to remove excess cement, which can still scratch the abutment, resulting in accumulation of plaque.15 Other techniques proposed have been to reduce the amount of cement used14 or to create a lingual vent hole.16
One other factor that must be considered when deciding on the method of retention is retrievability.17 Having access to the fixation screw that retains the abutment is important. For a number of reasons, abutment retentive screws can loosen, which mandates the separation of the restoration from the abutment to tighten this screw; therefore, the use of a provisional type of cement provides retrievability.18 However, in situations in which the vertical height of the abutment is less than 5 mm, which is required for retention and resistance form,19 a provisional type of cement may not afford sufficient retention and a more permanent type of cement must be utilized. The use of permanent cement then decreases the predictability of retrieval. In those cases with permanent cement, a loosened retaining screw can be accessed through a hole created from an occlusal approach. The initial entry through the crown is usually in a general location, and excessive crown material removal may occur and significantly lower the overall strength of the restoration.
Implant-Supported Crown Restorative Material
As previously mentioned, the successful long-term survival of dental implants is multifactorial. A number of authors have considered occlusal load to be a viable factor in the success of a dental implant.20-22 In a natural-tooth scenario, there is a semi-elastic, indirect connection between the tooth and surrounding alveolar bone, whereas, in the implant scenario, a rigid and direct connection exists between the implant fixture and surrounding alveolar bone. The periodontal ligament acts as a shock absorber for occlusal forces,23,24 which is not present with implants. The direct transmission of occlusal forces can have an adverse effect on the implant and peri-implant bone.25,26 Misch et al27 conducted an extensive literature review of articles that addressed biomechanical stress in bone loss at a cellular level, engineering principles, mechanical properties of bone, animal studies, bone physiology, and the biomechanics of implant design. They found the literature revealed occlusal overload on implants may increase the occurrence of marginal bone loss. On the contrary, Chang et al28 reviewed the available evidence regarding the impact of excessive occlusal forces on the peri-implant bone. They determined the number of studies was limited and the data presented, including animal studies, were conflicting. It was unclear whether occlusal overload had a detrimental effect on the bone surrounding an implant. The relationship of occlusal trauma and peri-implant bone loss remains controversial.29
Defining Occlusal Overload
The actual amount of force that would constitute an “overloading force” has never been quantified. Duyck and Vandamme30 conducted a literature review regarding the effect of occlusal overload. They observed these studies shared a lack of an assigned quantitative number to describe occlusal forces considered to be in excess of a normal load. Menini et al31 suggested the difficulty in defining a force to be considered excessive, or “overloading,” is because of the variability in the adaptability of the host physiologic response. They suggested overload can be considered as the amount of force that supersedes the adaptability potential of the host. In other words, it is difficult to quantify occlusal overload.
Restorative Materials and Transmission of Occlusal Forces
Occlusal forces are transmitted to the implant fixture through a rigid connection of the affixed restoration. The question remains as to how much of a role the restorative material plays in the amount of force transmission. Numerous studies in the last 20 years have repudiated the existence of the “shock absorbing” ability of resilient restoratives.32-37 However, Menini et al31 investigated how different restorative materials affected stress transmission on simulated peri-implant bone. They found materials with a lower elastic modulus (less rigid) recorded less stress. Bijjargi and Chowdhary38 used a 2D finite element model of a dental implant/abutment/crown assembly to measure stress dissipation from full-monolithic crowns made of zirconia, all-ceramic, metal, composite, and acrylic materials. They concluded the crown material with the lowest elastic modulus dampened the occlusal impact force the most while the zirconia exhibited the highest stress value.
Because the cause and effect of unquantified occlusal trauma or load is debatable, the author believes a material with a lower modulus should be used for implant-supported restorations, provided the esthetic demand is achieved. However, in the anterior region, where the planned implant restoration is adjacent to all-ceramic restored teeth, then it may be necessary to use the same restorative ceramic material with matching refractive indices to achieve an esthetic match.39
Low-Elastic-Modulus CAD/CAM Restoratives
Several low-elastic-modulus computer-aided design and computer-aided manufacturing (CAD/CAM) materials options are available and can be divided into two categories depending on their microstructure: composites with dispersed fibers and polymer-infiltrated-ceramic-network (PICN) materials. Paradigm MZ100 (3M ESPE, 3m.com) was the first commercial composite CAD/CAM milling block that contained zirconia-silica fillers (85 wt%) in a bisphenol A glycidylmethacrylate and triethylene glycol dimethacrylate (TEGDMA) matrix. The same manufacturer then introduced a material with a changed formulation—the “nanoceramic” version called Lava Ultimate. This product consisted of 79 wt% of zirconia-silica nanofillers and a urethane dimethacrylate (UDMA).
In 2012, the first PICN material (Vita Enamic, Vita Zahnfabrik, vita-zahnfabrik.com) was introduced. Using a polymer-infiltration manufacturing process, this material consists of a porous presintered glass-ceramic network infiltrated with a mixture of TEGDMA and UDMA.40 To avoid the formation of voids and poor filler distribution, high heat and pressure are incorporated into the manufacturing, which essentially involves taking a porous ceramic framework, infiltrating it with a liquid monomer, and polymerizing the organic component inside the ceramic structure under high temperature and heat.41 It differs from the aforementioned restoratives in which the filler material is in the form of dispersed or aggregated particles.39 The internal arrangement of PICN is a 3D interconnected skeleton,42 which is able to evenly distribute stress more effectively, thereby resisting crack propagation.43
The PICN CAD/CAM material has an elastic modulus closer to dentin than ceramics.44 For example, the elasticity (lower modulus) of Vita Enamic is a result of the organic polymer matrix, which is 14% by weight. The strength and stability of the millable material are derived from the inorganic ceramic network, which is 86% by weight.45 An internal study by the manufacturer found the product’s fracture load (2890 ± 232 N) was greater when compared with lithium disilicate CAD (2576 ± 206 N). Swain et al46 investigated the structure and some of the properties of the PICN millable material. They determined the lower-elastic-modulus materials were more damage tolerant than present glass-ceramic materials. In chewing simulations, they found the PICN crowns appeared to be more resistant to sliding/impact-induced cracking.
A clinical advantage for using PICN material for implant-supported crowns is the ability to add composite material post-milling. It is sometimes necessary to increase the interproximal contacts or, as in the following technique, adding composite to the undersurface of the restoration. This is not possible with a zirconia-based restoration. To improve the bonding surface of the PICN material, the manufacturer recommends 5% hydrofluoric-acid etching for 60 seconds instead of using air abrasion. The acid will dissolve the ceramic phase of the material that results in a “honeycomb” structure formed by the remaining resin network. The etched surface has high micromechanical interlocking potential for the addition of resin composite.39
Cement- and Screw-Retention Methodologies
The following technique describes a combination of both cement-retained and screw-retained methods of implant-supported restorations. This procedure can be used whether the restoration is fabricated in the laboratory or chairside employing CAD/CAM technology. The choice of restorative material is important when utilizing this cement-screw retention technique. Essentially, the restoration is permanently cemented to the abutment but can be retrieved by access to the retaining screw through a hole that was created during the fabrication of the restoration.
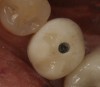
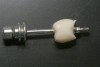
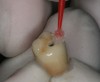
To begin, the implant fixture is uncovered, preferably with the use of a diode laser. A stock abutment that can be prepped is placed onto the implant fixture. A radiographic scan is taken to confirm the complete seating of the abutment. The necessary occlusal clearance, which is restorative material dependent, is marked with a notch. The interproximal clearances between the adjacent teeth are also evaluated and marked if any alteration is necessary. The abutment is then removed and modified where indicated. Once the spatial requirements are completed, cement retention undercuts are placed on the abutment. Using a carborundum disk or a No. 330 carbide bur, a circumferential undercut, 0.25 mm to 0.50 mm in depth, is placed toward the shoulder platform on the abutment (apical), and then another undercut is made 1 mm to 2 mm coronal to the first one (Figure 1). Depending on the implant system, sometimes the carrier, which is used to hold the implant during the surgical step, can be used in lieu of purchasing a separate abutment. One particular manufacturer uses grade 6 titanium in its carrier, which is not only compatible but also has the strength of a stock abutment. This particular carrier is designed with a large 360° undercut (Figure 2).
When the retentive undercuts are machined into the stock abutment, the anodized gold is removed. Depending on the location of the restoration, the loss of this color in the retentive undercuts may be esthetically undesirable. Or, when a titanium-colored abutment or carrier is used in the esthetic zone, the gray metallic hue may be difficult to mask by the restoration, causing an unfavorable esthetic result. To prevent a metallic hue, an electrochemical anodization technique can be used to alter the abutment surface and eliminate the potential of an undesirable color.47
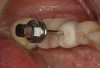
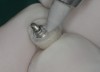
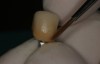
A commercially available titanium-anodizing unit can be used to apply a thin gold-colored film to mask the gray. The anodizing unit generates volts of direct current (VDC). When the metal abutment is placed into an acidic electrolyte solution (trisodium phosphate [TSP]), electrons are deposited onto the titanium surface (Figure 3 and Figure 4). The yellow gold is achieved at 60 VDC. If desired, a pink gingival color can be applied at the base of the abutment using 80 VDC to 85 VDC. Once the selected voltage is reached, the electron deposition stops and the abutment is removed from the solution and washed with deionized water.47,48
Scanning/Impression Step
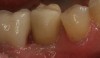
The altered, color-corrected abutment is then placed onto the implant fixture and torqued to the implant manufacturer’s recommendation. From an occlusal view, any gingival tissue that obstructs the margin of the abutment must be removed, preferably with the use of a diode laser. Placing block-out material into the screw-access hole of the abutment is not advised. Whether a CAD/CAM chairside system is used or a traditional impression technique is employed, leaving the screw-access hole open on the abutment will create a positive mark on the undersurface of the milled crown (Figure 5). This will aid in the precise placement of the screw-access hole. The steps in fabricating a CAD/CAM crown can be completed.
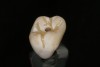
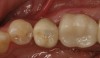
The milled crown is then tried in to verify the fit and occlusion. From the undersurface of the crown, the screw-access hole is located by identifying the extended portion that remained after milling (Figure 6). Using a 0.018-round tapered diamond bur, a hole is placed starting from the internal aspect of the crown surface and carried through the occlusal surface (Figure 7). The access hole is then refined from the occlusal side of the crown with a round diamond bur. The crown is then placed onto the abutment (Figure 8). From an occlusal view, the alignment of the access hole and implant screw is confirmed (Figure 9). The diameter of the hole is enlarged so that the head of the screw can pass through the opening. At this point, the crown can be either cemented onto the abutment or finished, stained, and glazed before cementation.
Crown Stain and Glazing Prior to Cementation
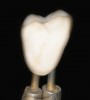
Once the occlusion has been verified and screw-access hole has been machined, the crown can now be stained and glazed. To avoid any contaminants from human contact with the crown during the following procedure, it is preferred to use a laboratory crown holder. The crown surface is conditioned first, preferably by the application of 5% hydrofluoric acid for 60 seconds (Figure 10). The alternative method of surface conditioning would be the use of 50-μm aluminum oxide air-abrasion under light pressure (14 psi). The surface is then silanated with a ceramic primer containing a 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) functional monomer.
The stains from the kit are mixed (using a nonmetallic spatula) to the preferred level of saturation (chroma). After mixing, the operator has approximately 10 minutes of working time because the stains have dual-cure chemistry.
The stains can be applied using the brushes supplied in the kit (Figure 11 and Figure 12). During the staining, an individual stain can be “set” in place with a few seconds of light exposure from a curing light. Once the staining is completed, the entire restoration is light polymerized before applying the final sealing glaze.
The last step is to glaze the entire restoration, which is important because this seals the underlying stains and provides a protective layer from the oral environment. A thin layer of the liquid glaze can be applied with a microbrush and then light polymerized (Figure 13).
Crown Cementation
Before the crown is cemented, a small piece of cotton or Teflon tape is placed in the screw hole of the abutment to keep cement from obstructing access to the abutment screw. The intaglio surface of the crown is etched for 60 seconds with 5% hydrofluoric acid. After rinsing and drying, the above-mentioned type of ceramic primer is applied. The abutment is cleaned and dried. To increase the bond strength of the resin cement to the titanium abutment, the same ceramic primer is applied to the abutment.49 Dual-cure resin cement is mixed and placed in the crown, which is seated while keeping the index finger over the screw-access hole to retain the cement. Once the crown is seated in place, a microbrush is used to clean out the excess resin cement in the screw-access hole. After the buccal and lingual sides of the crown are light polymerized for 10 seconds, cement in the screw-access hole is removed. The Teflon tape is then removed, exposing the head of the screw. The hex wrench is inserted, and the abutment screw is disconnected, allowing the crown/abutment assembly to be removed from the implant fixture (Figure 14).
The time it takes to re-cover and remove the abutment screw—the initial set time of the chemical-cure portion of the resin dual-cure cement (in addition to the 10-second light polymerization)—provides sufficient retention to remove the crown/abutment assembly intact. If the resin cement is fully polymerized and remains in an interproximal undercut, it may be more difficult to remove.
Once the crown/abutment assembly is out, the remaining excess cement is removed. The tissue side of the crown and crown/abutment interface can now be modified with the addition of resin composite. Any gaps between the crown and abutment can be filled. Adaptation of the underside of the crown to the gingival tissue and interproximal contacts, if necessary, are customized. The tissue surface is then finished and polished (Figure 15).
As previously mentioned, the stain-and-glaze steps can be completed after the crown is cemented to the abutment. In that case, the crown can be held by abutment and screw instead of laboratory crown holders while applying the stain and glaze (Figure 16 and Figure 17). The last stage is to apply glaze to the tissue surface and light polymerize (Figure 18).
The cemented implant crown, which has now been converted to a screw-retained version, can be placed on the implant fixture. The screw is then tightened to the manufacturer’s recommended amount of torque. A piece of Teflon tape or cotton is placed into the screw-access hole. The same ceramic primer is positioned around the opening of the screw-access hole and dried followed by the application of an adhesive bonding agent. The adhesive is then light polymerized followed by the application of a resin composite that matches the shade of the crown. After it is light polymerized, the occlusion of the resin composite “patch” is verified and then smoothed and polished (Figure 19 through Figure 21).
Conclusion
This simple, low-cost technique of converting a cement-retained implant crown to a screw-retained implant crown allows for easy retrievability and eliminates a number of laboratory steps and costs. For clinicians using a chairside milling system, additional scan bases and posts are no longer necessary to manufacture a screw-retained implant crown. This technique, using a lower-modulus PICN-type restorative material that allows modifications to be made easily, can fabricate an esthetically pleasing, low-occlusal–impact restoration. The screw-retained implant crown alleviates the concern of residual cement that can lead to peri-implantitis.
About the Author
Gregg A. Helvey, DDS, MAGD, CDT
Adjunct Associate Professor
Virginia Commonwealth University School of Dentistry
Richmond, Virginia
Queries to the author regarding this course may be sent to authorqueries@aegiscomm.com.
References
1. Chee W, Jivraj S. Failures in implant dentistry. Brit Dent J. 2007;202 (3):123–129.
2. Singer A, Serfaty V. Cement-retained implant-supported fixed partial dentures: a 6-month to 3-year follow up. Int J Oral Maxillofac Implants. 1996;11(5):645–649.
3. Shadid R, Sadaqa N. A comparison between screw- and cement-retained implant prostheses. A literature review. J Oral Implantol. 2012;38(3):298–307.
4. Michalakis KX, Hirayama H, Garefis PD. Cement-retained versus screw-retained implant restorations: a critical review. Int J Oral Maxillofac Implants. 2003;18(5):719–728.
5. Avivi-Arber L, Zarb GA. Clinical effectiveness of implant-supported single-tooth replacement: the Toronto study. Int J Oral Maxillofac Implants. 1996;11(3):311–321.
6. Hebel KS, Gajjar RC. Cement-retained versus screw-retained implant restorations: achieving optimal occlusion and esthetics in implant dentistry. J Prosthet Dent. 1997;77(1):28–35.
7. Misch CE. Dental Implant Prosthetics. St. Louis, MO: Mosby; 2005:414–420.
8. Chee W, Felton DA, Johnson PF, Sullivan DY. Cemented versus screw-retained implant prostheses: which is better? Int J Oral Maxillofac Implants. 1999;14(1):137–141.
9. Vigolo P, Givani A, Majzoub Z, Cordioli G. Cemented versus screw-retained implant-supported single-tooth crowns: a 4-year prospective clinical study. Int J Oral Maxillofac Implants. 2004;19(2):260–265.
10. Zarone F, Sorrentino R, Traini T, et al. Fracture resistance of implant-supported screw- versus cement-retained porcelain fused to metal single crowns: SEM fractographic analysis. Dent Mater. 2007;23(3):296–301.
11. Papavasileiou D, Behr M, Gosau M, et al. Peri-implant biofilm formation on luting agents used for cementing implant-supported fixed restorations: a preliminary in vivo study. Int J Prosthodont. 2015;28(4):371–373.
12. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388–1392.
13. Pauletto N, Lahiffe BJ, Walton JN. Complications associated with excess cement around crowns on osseointegrated implants: a clinical report. Int J Oral Maxillofac Implants. 1999;14(6):865–868.
14. Dumbrigue HB, Abanomi AA, Cheng LL. Techniques to minimize excess luting agent in cement-retained implant restorations. J Prosthet Dent. 2002;87(1):112–114.
15. Agar JR, Cameron SM, Hughbanks JC, Parker MH. Cement removal from restorations luted to titanium abutments with simulated subgingival margins. J Prosthet Dent. 1997;78(1):43–47.
16. Schwedhelm ER, Lepe X, Aw TC. A crown venting technique for the cementation of implant-supported crowns. J Prosthet Dent. 2003;89(1):89–90.
17. Gultekin P, Gultekin BA, Aydin M, Yalcin S. Cement selection for implant-supported crowns fabricated with different luting space settings. J Prosthodont. 2013;22(2):112–119.
18. Kurtzman GM. Crown and bridge temporization. Part II: provisional cements. Inside Dent. 2008;4(9).
19. Kaufman EG, Coelho AB, Colin L. Factors influencing the retention of cemented gold castings. J Prosthet Dent. 1961;11(3):487–502.
20. Isidor F. Influence of forces on peri-implant bone. Clin Oral Implants Res. 2006;17(suppl 2):8–18.
21. Miyata T, Kobayashi Y, Araki H, et al. The influence of controlled occlusal overload on peri-implant tissue. Part 3: a histologic study in monkeys. Int J Oral Maxillofac Implants. 2000;15(3):424–431.
22. Duyck J, Rønold HJ, Van Oosterwyck H, et al. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: an animal experimental study. Clin Oral Implants Res. 2001;12(3):207–218.
23. Matsuo M, Takahashi K. Scanning electron microscopic observation on microvasculature in the periodontium. Microsc Res Tech. 2002;56(1):3–14.
24. Skalak R. Biomechanical considerations in osseointegrated prostheses. J Prosthet Dent. 1983;49(6):843–848.
25. Tawil G. Peri-implant bone loss caused by occlusal overload: repair of the peri-implant Defect following correction of traumatic occlusion. A case report. Int J Maxillofac Implants. 2008;23(1):153–157.
26. Fu JH, Hsu YT, Wang HL. Identifying occlusal overload and how to deal with it to avoid marginal bone loss around implants. Eur J Oral Implantol. 2012;(5 suppl):S91–S103.
27. Misch CE, Suzuki JB, Misch-Dietsh FM, Bidez MW. A positive correlation between occlusal trauma and peri-implant bone loss: literature support. Implant Dent. 2005;14(2):108–116.
28. Chang M, Chronopoulos V, Matteos N. Impact of excessive occlusal load on successfully-osseointegrated dental implants: a literature review. J Investig Clin Dent. 2013;4(3):142–150.
29. Junqueira RB, Saaveda FA, de Siqueira G, de Macedo NL. Considerations about the relation between occlusal trauma and periodontal/peri-implant disease. Braz Dent Sci. 2015;18(2):9–14.
30. Duyck J, Vandamme K. The effect of loading on peri-implant bone: a critical review of the review. J Oral Rehabil. 2014;41(10):783–794.
31. Menini M, Conserva E, Tealdo T, et al. Shock absorption capacity of restorative materials for dental implant prostheses: an in vitro study. Int J Prosthodont. 2013;26(6):549–556.
32. Stegaroiu R, Khraisat A, Nomura S, Miyakawa O. Influence of superstructure materials on strain around an implant under 2 loading conditions: a technical investigation. Int J Oral Maxillofac Implants. 2004;19(5):735–742.
33. Bassit R, Linström H, Rangert B. In vivo registration of force development with ceramic and acrylic resin occlusal materials on implant-supported prostheses. Int J Oral Maxillofac Implants. 2002;17(1):17–23.
34. Wang TM, Leu LJ, Wang JS, Lin LD. Effects of prosthesis materials and prosthesis splinting on peri-implant bone stress around implants in poor-quality bone: a numeric analysis. Int J Maxillofac Implants. 2002;17(2):231–237.
35. Soumeire J, Dejeo J. Shock absorbability of various restorative materials used on implants. J Oral Rehabil. 1999;26(5):394–401.
36. Sertgoz A. Finite element analysis study of the effect of superstructure material on stress distribution in an implant-supported fixed prosthesis. Int J Prosthodont. 1997;10(1):19–27.
37. Papavasiliou G, Kamposiora P, Bayne SC, Felton DA. Three-dimensional finite element analysis of stress-distribution around single tooth implants as a function of bony support, prosthesis type, and loading during function. J Prosthet Dent. 1996;76(6):633–640.
38. Bijjargi S, Chowdhary RS. Stress dissipation in the bone through various crown materials of dental implant restoration: a 2-D finite element analysis. J Investigat Clin Dent. 2012;4(3):122–177.
39. Mainjot AK, Dupont NM, Oudkerk JC, et al. From artisanal to CAD-CAM blocks: state of the art of indirect composites. J Dent Res. 2016;95(5):487–495.
40. Nguyen JF, Ruse D, Phan AC, Sadoun MJ. High-temperature-pressure polymerized resin-infiltrated ceramic networks. J Dent Res. 2014;93(1):62–67.
41. Franco Steier VF, Koplin C, Kailer A. Influence of pressure-assisted polymerization on the microstructure and strength of polymer-infiltrated ceramics. J Mater Sci. 2013;48(8):3239–3247.
42. Harris JJ, Marquis PM. Comparison of the deformation and failure characteristics of morphologically distinct metal-glass interpenetrating phase composites. J Mater Science. 2002:37(13);2801–2810.
43. Clarke DR. Interpenetrating phase composites. J Amer Cer Soc 1992;75(4):739–759.
44. Coldea A, Swain MV, Thiel N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent Mater. 2013;29(4):1405–1411.
45. Leung BT, Tsoi JK, Matinlinna JP, Pow EH. Comparison of mechanical properties of three machinable ceramics with an experimental flurophlogopite glass ceramic. J Prosth Dent. 2015;114(3):440–446.
46. Swain MV, Coldea A, Bilkhair A, Guess PC. Interpenetrating network ceramic-resin composite dental restorative materials. Dent Mater. 2016;32(1):34–42.
47. Wadhwani CPK, O’Brien R, Kattadiyil MT, Chung KH. Laboratory technique for coloring titanium abutments to improve esthetics. J Prosthet Dent. 2016;115(4):409–411.
48. Kern P, Schwalier P, Michler J. Electrolytic deposition of titania films as interference coating on biomedical implants: microstructure, chemistry and nano-mechanical properties. Thin Solid Films. 2006;494(1):279–286.
49. Blatz MB, Sadan A, Martin J, Lang B. In vitro evaluation of shear bond strengths of resin to densely-sintered high purity zirconium oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004;91(4):356–362.