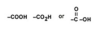You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Disclaimer: Participants must always be aware of the hazards of using limited knowledge in integrating new techniques or procedures into their practice. Only sound evidence-based dentistry should be used in patient therapy.
Introduction
The course will focus on the most common dentifrice ingredients and the oral health benefits they provide. Upon completion of the course, participants will understand not only the fundamentals of dentifrice ingredients, but also key regulatory aspects of the dentifrice market and the role of professional societies in credentialing consumer dentifrices.
Overview
The majority of patients use dentifrice in their daily hygiene routine. As such, it is a cost-effective and convenient vehicle to deliver ingredients that provide therapeutic benefits, cosmetic benefits, or both. Classification of dentifrice ingredients into these key benefit categories affects how products are regulated as well as the types of claims that can be made about a product. Products providing therapeutic benefits are regulated by the US Food and Drug Administration (FDA).
The first therapeutic ingredient to be included in dentifrices was fluoride. Since this first therapeutic advancement in the dentifrice market, many other ingredients have been formulated into dentifrices to provide other benefits, such as plaque and gingivitis reduction, enamel protection, antihypersensitivity benefits, extrinsic whitening, calculus protection and reducing bad breath. These ingredients and their mechanisms of action are described in detail in this course, along with ingredients that provide stability and esthetic benefits to a dentifrice formulation.
Dentifrice Market Fundamentals
The use of dentifrice as part of daily hygiene in the United States is widespread. In fact, there are so many dentifrice options in the oral care aisle today it can be overwhelming. Patients often turn to dental professionals for a product recommendation that will meet their specific oral care needs and desires. Understanding the regulatory environment that guides product claims as well as the process used by credentialing bodies to evaluate products is important as professionals discuss home care options with patients.
In the United States, the Food and Drug Administration (FDA) regulates therapeutic agents to ensure product safety and efficacy. Drugs can enter the market by one of two regulatory pathways. The most common pathway for over-the-counter (OTC) drugs is under the OTC Monograph system. There are three monographs that regulate OTC dentifrices.
The second pathway is through a new drug application (NDA), which is used for new drug products that fall outside the range of ingredients already included in the OTC Monograph system. NDAs for dentifrices are uncommon, and require the manufacturer to demonstrate that the product is safe and effective through comprehensive clinical testing.
The American Dental Association (ADA), as the leading US dental professional association, takes an interest in educating the public on the safety and efficacy of oral health products. Its primary mechanism for this is by awarding the ADA Seal of Acceptance to qualifying products.
Description of the US Monograph System
In 1962, an amendment was passed to the US Federal Food, Drug, and Cosmetics Act (FD&C) requiring that marketed drug products not only had to be safe, but they also had to be effective. At that time, hundreds of thousands of OTC drugs were on the market, and time and resources were too limited to ensure that all these OTC drugs complied with the new regulations.
To ease the approval of OTC therapies considered safe by virtue of their extensive historical use, the monograph drug review mechanism was instituted in 1972. Under this process, the FDA convenes committees to review safety and efficacy data submitted for therapeutic ingredients in the OTC market. The end result of the process is a published document that lists certain therapeutic ingredients (referred to as active ingredients) and the requirements for marketing products that contain those active ingredients. These requirements include a number of parameters, including the intended use, drug dosage or concentration, dosage forms, allowable combinations with other drugs, required labeling, and any special packaging or testing requirements.1
There are several classes of monographed OTC drugs for oral use, including anticaries agents, tooth desensitizers, oral antiseptics, anesthetics, and analgesics. Therapeutic dentifrices are regulated by three separate monographs: Anticaries, Antiplaque-Antigingivitis, and Tooth Desensitizer (Table 1).
One factor that differentiates OTC fluoride dentifrices from prescription fluoride dentifrices is the amount of fluoride they contain as a therapeutic ingredient. Fluoride is a known anticaries ingredient, but it can be toxic if excessive levels are ingested. Although dentifrices are not intended to be ingested, there is enough safety concern to warrant stricter regulations for higher dose products. Most OTC fluoride dentifrices contain 1000-1500 parts per million (ppm) of fluoride and are considered conventional fluoride dentifrices. The maximum allowable fluoride in a monographed OTC dentifrice is 1500 ppm for a sodium monofluorophosphate dentifrice and 1150 ppm for sodium fluoride and stannous fluoride dentifrices. Some prescription-strength fluoride dentifrices contain as much as 5000 ppm of fluoride. Because this level of fluoride is not allowed in an OTC product under the anticaries monograph, these types of products must be prescribed by a dentist.2,3
Monograph vs. New Drug Application (NDA) for Marketing Approval
Drug products that do not meet the specific monograph criteria are subject to approval through a New Drug Application (NDA). For an NDA, extensive data packages (e.g., clinical efficacy, pharmacology and toxicology data) must be submitted for FDA review to establish the drug's safety and efficacy to receive marketing approval. Important characteristics of NDA and monographed drugs are presented in Table 2.4
If a product contains a drug not included in the monograph, it must be approved through the NDA process. Even if the drug is included in the monograph but is being used at a different dose, for a new indication, or in combination with another drug (dual-active product) not specified in the monograph, the product is subject to NDA approval. For example, triclosan is an antibacterial ingredient that is not included in the Antiplaque-Antigingivitis Monograph. Colgate® Total®, a fluoride dentifrice containing triclosan, required approval through an NDA before it could be marketed in the US as an antibacterial dentifrice that treats gingivitis.
Despite their differences, both NDAs and monographs for OTC medicines have very similar standards for safety and efficacy.4
Claims for Therapeutic vs. Cosmetic Benefits
The US Federal Food Drug & Cosmetic Act defines a cosmetic as an article intended to be applied to the human body to cleanse, beautify, promote attractiveness, or alter the appearance. In contrast, a therapeutic drug is defined as an article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease, or article intended to affect the structure or any function of the body. Manufacturer claims for therapeutic vs. cosmetic benefits thus have to follow these definitions.1
The key therapeutic areas for dentifrices are caries, gingivitis and sensitivity. In addition, several oral care products are marketed with cosmetic claims such as whitens teeth, reduces bad breath, and protects against tartar. If an ingredient is not included in an OTC monograph or is not approved under an NDA, it is not considered a drug, and therapeutic claims cannot be made for it. Many nontherapeutic ingredients are described later in this course.1,3
Credentialing Bodies
While not a regulatory body, the American Dental Association (ADA) takes a vested interest in informing the public on the safety and effectiveness of oral care products. They do this primarily through their Seal of Acceptance program, which began in 1930. The ADA Seal of Acceptance program is entirely voluntary, whereby manufacturers may submit safety and efficacy data for a product to the ADA Council on Scientific Affairs. Through a review process, the Council decides whether to award its Seal of Acceptance to the product.5
The ADA Seal of Acceptance can be a powerful product endorsement in both professionals' and consumers' minds, as both of these groups have come to trust the ADA for providing guidance on the safety and efficacy of products. In order to obtain the ADA Seal, manufacturers are required to submit data in accordance with published ADA guidelines for confirmation of each benefit for which the Seal of Acceptance is desired. For some benefits, these requirements include the submission of at least two well controlled clinical studies confirming efficacy, with the clinical trials run according to established ADA protocols. For benefits where clinical studies are not required, the ADA has established a series of specific laboratory tests that must be followed in order to confirm product effectiveness. For a product that wishes to claim multiple benefits, each benefit must be confirmed according to the required guidelines; thus, the overall investment (both clinical and laboratory) to obtain the ADA Seal is considerably higher for a product that is able to claim multiple benefits, compared to a product that claims a single benefit. The ADA Seal is usually awarded for a 5-year period, after which the company must seek renewal. If a dentifrice formulation changes, the manufacturer must submit a new application for the modified product.5,6
Click here for more information about the "ADA Seal of Acceptance Program & Products" requirements and products that carry the ADA Seal.
Dentifrice Ingredients Providing Therapeutic or Cosmetic Benefits
Dentifrices contain ingredients that help reduce caries, plaque, gingivitis, hypersensitivity, dental erosion, calculus, stain, and halitosis. Some ingredients provide a therapeutic benefit, while other ingredients or additives contribute to the cosmetic benefits or physical properties of the dentifrice.
The first dentifrice ingredient clinically proven to provide a health benefit was fluoride, which can be delivered from one of several different fluoride-based compounds (three are allowed for use in the US under the US monograph system). Over time, dentifrices evolved to provide multiple therapeutic and cosmetic benefits. This section of the course describes the most common dentifrice ingredients used for therapeutic benefits (caries, plaque/gingivitis, and hypersensitivity) as well as cosmetic ones (calculus, whitening, and bad breath), and it provides perspective on how the market evolved to deliver multiple benefits in dentifrice formulations.
Caries Prevention - Fluorides
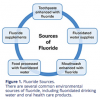
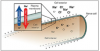
It is widely accepted that the regular use of fluoride, such as in dentifrice and drinking water, is extremely effective at preventing dental caries. In 1999, the US Center for Disease Control (CDC) issued a statement that water fluoridation is one of the 10 most important public health measures of the 20th century.7 Fluoride's presence in low concentration and high frequency is more effective at preventing caries than high levels of fluoride used in low frequency. Because water fluoridation is not available in many countries, dentifrice is considered to be one of the most important sources of fluoride globally.8 Common environmental sources of fluoride are depicted in (Figure 1).2,9,10
Successfully formulating a fluoride dentifrice that was efficacious against caries was a significant oral health breakthrough, because fluoride is incompatible with many dentifrice ingredients or additives. In 1950, The Procter & Gamble Company formed a joint research project team headed by Dr. Joseph Muhler at Indiana University to develop and test a new dentifrice with fluoride. Results from a clinical study of this dentifrice indicated that children ages six to 16 showed an average 49% reduction in cavities, and adults showed tooth decay reduction to almost the same degree.11,12
Following the success of this study, Crest® with Fluoristan® dentifrice launched into a number of test markets in 1955, followed by national expansion in January, 1956. In 1960, and again in 1964, the American Dental Association confirmed that Crest effectively prevents tooth decay, reporting that "Crest has been shown to be an effective anticavity dentifrice that can be of significant value when used in a conscientiously applied program of oral hygiene and regular professional care" in granting its Seal of Acceptance (Figure 2).13,14 In 1976, the American Chemical Society recognized Crest with fluoride as one of the 100 greatest discoveries of the previous 100 years.15
The following sections will explain the mechanism of action of fluoride, and common fluorides in dentifrices will be described.
Fluoride's Mechanism of Action. Dental caries is an infectious disease caused by the complex interaction of cariogenic (caries-causing) bacteria with carbohydrates (i.e., sugars) on the tooth surface over time. Cariogenic bacteria metabolize carbohydrates for energy and produce organic acids as byproducts. The acids lower the pH in the plaque biofilm.16
The hydroxyapatite of tooth enamel is primarily composed of phosphate ions (PO43-) and calcium ions (Ca2+). Under normal conditions, there is a stable equilibrium between the calcium and phosphate ions in saliva and the crystalline hydroxyapatite that comprises 96% of tooth enamel. When the pH drops below a critical level (5.5 for enamel, and 6.2 for dentin), it causes the dissolution of tooth mineral (hydroxyapatite) in a process called demineralization. When the natural buffer capacity of saliva elevates pH, minerals are reincorporated into the tooth through the process of remineralization (Point of Interest, Figure 3).16-18
Caries is simply the result of a series of demineralization/remineralization cycles where, over time, demineralization conditions prevail. The caries process can be affected in several ways. One of the most effective methods to prevent caries is by promoting remineralization and slowing down demineralization. This can be accomplished with fluoride therapy.2,9,19
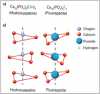
When fluoride is present in oral fluids (i.e., saliva), fluorapatite, rather than hydroxyapatite, forms during the remineralization process. Fluoride ions (F-) replace hydroxyl groups (OH-) in the formation of the apatite crystal lattice (Figure 4). In fact, the presence of fluoride increases the rate of remineralization.
Fluorapatite is inherently less soluble than hydroxyapatite, even under acidic conditions. When hydroxyapatite dissolves under cariogenic (acidic) conditions, if fluoride is present, then fluorapatite will form. Because fluorapatite is less soluble than hydroxyapatite, it is also more resistant to subsequent demineralization when acid challenged (Figure 5).
Caries is a sub-surface phenomenon. With fluoride treatment, a noncavitated lesion can be remineralized with fluorapatite and have greater resistance to subsequent demineralization than hydroxyapatite (Figure 6). Even when available at very low concentrations, fluoride is effective as an anticaries agent.2,19,21
Video 1. Demineralization/Remineralization with Fluoride.
Common Fluorides. Fluoride can be delivered from several different fluoride sources. The three most popular sources of fluoride globally are:
• stannous fluoride (SnF2)
• sodium fluoride (NaF)
• sodium monofluorophosphate (Na2PO3F)
The efficacy of fluoride as a caries preventive agent depends largely on its concentration and availability in the oral fluids to affect the demineralization/remineralization balance. Over the years, hundreds of clinical studies have been conducted to test the efficacy of fluoride dentifrices in caries prevention. In general, across all fluoride types, these studies show approximately a 25% reduction in caries over a nonfluoridated control dentifrice.22
a. Stannous fluoride. Stannous fluoride (SnF2; also called tin fluoride) was first formulated successfully into a dentifrice to deliver an anticaries benefit in the 1950s.11,12 Fluoride is highly reactive, and the challenge was finding an abrasive system that had low enough reactivity with fluoride to maintain the bioavailability of the fluoride. The formulation included 0.454% stannous fluoride and the abrasive calcium pyrophosphate; it was marketed as Crest® with Fluoristan® (Figure 2). While stannous fluoride also has the potential to deliver benefits related to the antibacterial properties of the ingredient, this early formulation only delivered an anticaries benefit based on the action of fluoride. In the 1990s, manufacturers developed methods to stabilize stannous fluoride formulations that delivered the antibacterial benefit of the ingredient as well.23,24
b. Sodium fluoride. Sodium fluoride (NaF) is a fluoride salt commonly used in dentifrices and oral rinses. Sodium fluoride delivers a highly reactive fluoride ion; therefore, formulating it with a compatible abrasive is critically important for achieving the anticaries benefit. The earliest fluoride dentifrices, formulated with NaF and calcium abrasives, provided essentially no anticaries efficacy.25,26 In the early 1980s, silica abrasives that were compatible with sodium fluoride became available and allowed dentifrices with NaF to be developed; these formulas were tested and proven to be clinically effective against caries.27,28
c. Sodium monofluorophosphate. Sodium monofluorophosphate (SMFP) was introduced into Colgate's first fluoridated dentifrice and allowed this brand to obtain the ADA Seal of Acceptance for cavity protection in 1968 (Figure 7).29 Unlike sodium fluoride, SMFP is not an ionic fluoride salt, but rather a covalently bound compound that requires enzymatic activation by a salivary enzyme (alkaline phosphatase) to release bioavailable fluoride (Figure 8).30 Because of this lower reactivity, SMFP is compatible with more abrasives than other fluoride sources.29
Dental Erosion
Dental erosion is characterized by the dissolution and removal of the tooth enamel surface under highly acidic conditions. When the localized pH drops below approximately 4.5, the pellicle cannot protect the enamel surface, and irreversible erosive damage can occur. Enamel erosion has become an important issue with the increased consumption of sports drinks, soft drinks, and citric juices.31 All of these products have a pH below the critical level for dissolving dental enamel. In this context, the enamel erosion benefits of existing dentifrice ingredients has become more relevant and are described below.32‑24
Fluoride (in general). The mechanism by which fluoride helps prevent caries is the same mechanism by which it provides some dental erosion benefit. Fluoride exerts an effect by favoring remineralization and inhibiting demineralization.2,9,16
The presence of bioavailable fluoride in the oral fluids (i.e., biofilm and saliva) greatly enhances the crystallization of fluorapatite into tooth structure from calcium and phosphate ions present in saliva. Fluorapatite is more resistant to demineralization than hydroxyapatite, and thus provides some minimal level of enamel erosion benefit; but even fluorapatite will dissolve under highly acidic conditions.
Stannous Fluoride. In addition to the modest level of enamel erosion protection provided by fluoride in general, stannous fluoride is unique in its ability to provide significantly greater levels of protection compared to other fluoride sources.35-41 This is because stannous fluoride adheres to the surface of tooth enamel and forms a protective layer that is able to shield enamel from the effects of erosive acids (Figure 9).42-45
Antiplaque/Antigingivitis
Adding antibacterial action to dentifrice to reduce plaque and gingivitis was another major therapeutic breakthrough. Several modern dentifrice ingredients have the potential to kill or inhibit bacteria that cause plaque and gingivitis. The mechanism of action varies depending on the specific antibacterial ingredient.
Stannous Fluoride. Stannous fluoride exerts antibacterial effectsby two modes of action. First, stannous fluorideexerts a killing effect on bacteria (bactericidal action). This is probably due to non-specificinteraction with the bacterial membrane thatcauses membrane disruption. The result isleakage of cellular components that leads to celllysis and death.
The second, and more important, mode of antibacterial activity is through stannous fluoride's inhibition of metabolic enzymes. The inhibition of metabolic activity affects bacteria in a number of ways, including:46,47
• reduction of bacterial growth
• prevention of bacterial adhesion to oral surfaces (e.g., enamel, exposed dentin)
• reduction in bacterial byproducts that boost the inflammatory response leading to gingivitis
Stannous fluoride's inhibitory effect on bacteria is related to its inhibition of bacterial glycolysis, an energy making process whereby metabolic enzymes break down carbohydrates. In addition, studies have demonstrated that stannous fluoride significantly reduces metabolic toxins produced by bacteria in plaque biofilm.46,47
In early stannous fluoride dentifrice formulations, the stannous fluoride was not fully stable or bioavailable. A key issue in formulating with stannous fluoride is that it is easily inactivated by hydrolysis and oxidation, thus making it difficult to stabilize in a typical dentifrice formula. Stannous fluoride can also have an astringent taste and cause extrinsic staining of teeth and fillings. However, current stannous fluoride-containing dentifrices have been formulated that circumvent instability and bioavailability challenges.
One of the major breakthroughs in stannous fluoride formulation efforts was due to technology innovations that enabled the combination of both whitening and stabilization chemistries to provide highly effective stannous fluoride formulations that are not compromised by common esthetic negatives, such as poor taste or staining, of earlier stannous fluoride products.23,48,49 After more than fifty years of research, P&G successfully formulated the first stabilized, consumer-acceptable, stannous fluoride dentifrice with the launch of Crest® PRO‑HEALTH® dentifrice in 2005.23,24,48,49 Crest® PRO‑HEALTH® dentifrice (Figure 10) carries the ADA Seal of Acceptance in the therapeutic categories of caries, gingivitis and sensitivity.
Triclosan. Triclosan is a broad-spectrum antibacterial agent that inserts into and disrupts the bacterial membrane. Being a nonpolar molecule, it has an affinity for the hydrophobic environment of the lipid bilayer. This causes leakage of cellular components, ultimately leading to cell death.
Triclosan is the antibacterial ingredient in Colgate® Total®, and it provides the plaque and gingivitis benefits of the dentifrice. Since it is an uncharged molecule, triclosan itself has poor retention (substantivity) in the oral cavity (Figure 11).50-52 Colgate® Total® is formulated with a special polymer (Gantrez®), which increases the substantivity of triclosan in the oral cavity. Colgate® Total® was introduced outside the U.S. in 1992 and was the first broadly marketed antibacterial dentifrice. Because triclosan is not included in the US Antiplaque-Antigingivitis Monograph, Colgate® Total® had to be approved through an NDA before it could be sold in the US. It received US marketing approval in 1997. Colgate® Total® (Figure 12) carries the ADA Seal of Acceptance and has been demonstrated effective in the therapeutic categories of caries and gingivitis.53-55
Video 2. Progression of Gingivitis Induced by Bacteria.
Video 3. How an Antibacterial Agent Reduces Bacteria and Gingivitis (Inflammation).
Video 4. Fluid Movement in Tubules.
Antihypersensitivity
Cervical dentinal hypersensitivity is a condition characterized by sharp pain associated with thermal, evaporative, tactile, osmotic or chemical stimuli. This condition depends on dentin exposure, as well as the patency of the dentinal tubules. It is widely accepted that dentinal hypersensitivity is a result of fluid movement within the dentinal tubules, which stimulates nerve endings in the pulp matrix.56-60
Tooth hypersensitivity is a condition patients commonly report to their dental professional; thus, it is a segment of the dentifrice market heavily influenced by professional recommendations. It has been reported that up to 57% of the adult population suffers from this condition.56,61
A segment of the fluoride dentifrice market has emerged that specifically addresses the needs of patients suffering from sensitive teeth. One of the first dentifrice products to enter this segment of the market was Sensodyne®, which was introduced in 1961. More recently, tooth sensitivity has become a very dynamic area, as several new products have entered the market with proprietary ingredients to treat dentinal hypersensitivity.
As noted above, exposure of dentinal tubules to external stimuli is a common cause of tooth sensitivity. Dentinal hypersensitivity is generally treated in one of two ways.
1. Chemical desensitization of the tooth nerve endings (nerve depolarization).
2. Tubule occluding agents or barriers to reduce dentin permeability.
Antihypersensitivity treatments with these mechanisms are described below.
Nerve Depolarization Agents. To understand how a chemical desensitization agent works, one must first understand how a nerve cell transmits pain stimuli. Potassium (K+), sodium (Na+), and chloride (Cl-) ions are all involved in the electrical activity of nerve cells. When the nerve cell is at rest, the potassium ion concentration is higher on the inside of the cell than on the outside, while the sodium ion concentration is higher on the outside of the cell than on the inside (Figure 13). When the nerve cell is stimulated, these ions cross the nerve cell membrane through channels and move from an area of high concentration to an area of lower concentration (referred to as the concentration gradient). Thus, potassium ions flow from the inside to the outside of the cell and the sensation of pain is transmitted.
Potassium ion is a desensitization agent because it diffuses through dentin tubules and increases the extracellular potassium concentration at the nerve ending, eliminating the potassium ion concentration gradient across the nerve cell membrane. Without this concentration gradient, the nerve cell will not depolarize and will not respond to stimuli; thus the sensation of pain will not be transmitted. Potassium ion can be delivered in a variety of salt forms (e.g., potassium nitrate, potassium citrate). The most common potassium salt used in sensitivity dentifrices is potassium nitrate (KNO3).62
Video 5. How Nerve Depolarization Agents Work.
Tubule Blocking or Occluding Agents. Another strategy to treat/prevent dentinal hypersensitivity is to reduce the permeability of the dentin by occluding or blocking the exposed dentin tubules. This prevents stimuli from causing fluid flow in the tubules, thereby preventing the nerve endings inside the tooth from being stimulated.58,59,63
a. General mechanism of action. Several dentifrice ingredients can be used to occlude or block the dentinal tubules. All of these agents have similar mechanisms of action, forming salt precipitates on the surface of the exposed dentin and inside the dentinal tubules. These precipitates effectively reduce or block the fluid flow in the tubules and exert a desensitization effect. Strontium chloride was the desensitizing ingredient used in the original Sensodyne® dentifrice, and it acted via this mechanism by forming strontium salt precipitates; however, it is rarely used anymore because of its strong metallic taste and incompatibility with fluoride.
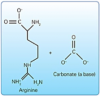
b. Newer tubule-occluding agents. Other tubule-occluding agents new to the market include arginine with calcium carbonate (Pro-Argin™), strontium acetate, and calcium sodium phosphosilicate (Novamin®).
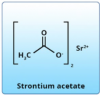
Strontium acetate. Unlike the original strontium chloride, strontium acetate (Figure 15) can be formulated into fluoride-containing dentifrices. Upon toothbrushing, strontium-based precipitates form to occlude dentinal tubules and build a resistant barrier over time.
Calcium sodium phosphosilicate (Novamin®). In saliva, Novamin® releases calcium andphosphate ions and raises the pH (Figure 16). Under these conditions, calcium phosphatesalts precipitate from solution to not onlyblock dentin tubules but also to form aninsoluble calcium phosphate layer on thesurface of enamel.58,65,66
c. Stannous fluoride. Stannous fluoride is also a tubule-occluding agent that can treat dentinal hypersensitivity. Stannous fluoride, through hydrolysis and oxidation reactions, forms many insoluble metal salts that can precipitate in dentinal tubules and on the dentin surface (Video 6) to provide effective relief against hypersensitivity.24,56,67 Stannous fluoride is the only fluoride delivering protection from caries68 and plaque/gingivitis47,69 as well as hypersensitivity.56,70
Video 6. Tubule Occlusion.
Cosmetic Benefits
While delivering fluoride for cavity protection was a major therapeutic advance in the dentifrice market, researchers saw an opportunity, over time, to expand the benefits offered by dentifrice. By the 1980s, additional innovations were having an impact. Agents were discovered that could provide protection against calculus and stain, and this opened an era where improved cosmetic benefits spurred the dentifrice market.
Calculus Control. Dental plaque calcifies when calcium phosphate begins depositing in it. Under normal conditions, the oral fluids are saturated with calcium and phosphate, which is important for maintaining sound enamel. However, this abundance of mineral ions also contributes to calculus formation on the tooth surface (i.e., calcification of plaque biofilm). The amount and type of calcium phosphate salts present vary greatly but include brushite, octacalcium phosphate (OCP), tricalcium phosphate (TCP) and apatite. While supragingival calculus forms from saliva, subgingival calculus forms either from saliva or crevicular fluid. Dental calculus that forms from crevicular fluid can contain heme and some breakdown products which make it pigmented. It is called serumnal calculus. Calculus forms most readily in areas which are adjacent to the openings of the salivary ducts, where the calcium phosphate in saliva is least stable. In populations with poor oral hygiene, supragingival calculus can be extensive and result in gingival recession. Calculus formation can be controlled by adding mineralization inhibitors to dentifrices and mouthrinse. The chemical agents used most often for calculus control in dentifrice are described briefly below.71
Video 7. Formation of Calculus.
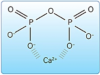
a. Pyrophosphate. Phosphate is a ubiquitous chemical group found in biological systems. As shown in (Figure 17), two phosphate groups combine chemically to form a molecule called pyrophosphate (P2O74-).
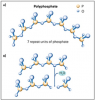
b. Sodium hexametaphosphate (SHMP). SHMP is a large polyphosphate molecule and has multiple calcium binding sites in one molecule. It is a very effective calculus inhibitor. Because it works only on the surface, it is sometimes called a calcium surface active builder. SHMP is susceptible to hydrolysis, and must be formulated in a low water dentifrice to be stable (Figure 20).74,75 SHMP particles will not dissolve in low-water formulations, so the SHMP particles may be perceived as "gritty." However, these particles are highly soluble in water and will begin dissolving immediately upon brushing without imparting abrasive action.
c. Zinc. Zinc salts (e.g., zinc citrate, zinc chloride, zinc lactate) are used in some tartar control dentifrices and oral rinses, and have been shown to be moderately effective at controlling calculus.76 Positively charged zinc ions (Zn2+) inhibit crystal growth by substituting for calcium in the crystal lattice of calcium phosphate. This interferes with the crystal formation and slows crystal growth. As a result, calculus formation is reduced.
d. Gantrez®. Gantrez® is a copolymer of methylvinyl ether (PVM) and maleic acid (MA) and is an ingredient in Colgate® Total® dentifrice. The mechanism of action for this co-polymer is to bind (chelate) calcium ions, thus inhibiting plaque mineralization (Figure 21).
Video 8. Mechanism for Calculus Protection.
Stain Control/Whitening Agents. Stain control and whitening are key benefits of modern dentifrices. These are accomplished via ingredients that target specific types of tooth stain. Stains can be classified as extrinsic (surface stains) or intrinsic (below the enamel surface), and their management is based primarily on that classification (Table 3). Dentifrices primarily work against extrinsic stains. Bleaching products that contain hydrogen peroxide (i.e., whitening strips) or carbamide peroxide (i.e., dental office bleaching trays) and allow longer contact time with the dentition address intrinsic stains as well as extrinsic stains.48,77
a. Extrinsic stain. Despite being extremely hard, the tooth's surface can be stained. Extrinsic (surface) stains can generally be relatively easily removed on a daily basis by proper tooth brushing with a dentifrice. If extrinsic stains are not frequently removed, they can firmly attach to the tooth surface, and may require a professional cleaning (prophylaxis) for their removal. Surface stains can be removed daily through either physical or chemical action, as described below.48,77
Physical action. Most dentifrices contain mild abrasives that help clean precipitated stain particles from the tooth surface, controlling surface stains with home care. While dentifrice abrasives are desirable to keep stains off teeth, they are designed to not wear down the tooth enamel over time with repeated brushing.78
The abrasivity of dentifrice is measured in terms of Relative Dentin Abrasivity, or RDA. This rating was introduced in the early 1970s and is used by professional dental societies and boards of health to rate the abrasivity of commercial dentifrices.78,79
RDA values are obtained in the laboratory by comparing the amount of tooth structure worn away by a standardized tooth brushing protocol using any given dentifrice with that of a standard dentifrice. The standard protocol integrates factors such as pressure, time, temperature, and humidity. Because dentifrices with lower RDA values are less abrasive, they also tend to remove less surface stain. The International Standards Organization (ISO) specification states that a dentifrice should not exceed an RDA of 250, which is considered safe for hard tissues for a lifetime of use. Although there is a wide range of RDA values for various dentifrices, there are no relative degrees of safety between 0 and 250. In other words, a dentifrice with an RDA of 200 is as safe as one with an RDA of 50 for daily usage. Having an effective abrasive system in a dentifrice is important for cleaning the teeth and removing extrinsic stain.80
Fluoride ions are very reactive and can interact with common dentifrice abrasives, rendering the fluoride inactive for caries control. Also, many dentifrice abrasives have a very porous, negatively charged surface that can bind many dentifrice ingredients (e.g., stannous), lowering their bioavailability. For this reason, formulating dentifrices with the right abrasive is critical to achieving the desired benefits of other ingredients (Table 4).
Chemical action. Polyphosphates, which are dentifrice ingredients used to control calculus, also target extrinsic stain. One of the most effective ingredients with this dual action is SHMP, which is used in several marketed dentifrices. SHMP controls stain with a chemical action. Stain molecules, or chromogens, are usually negatively charged molecules; they have an affinity for positively charged ions like calcium (Ca2+) that reside in the tooth enamel and crosslink pellicle proteins. SHMP is also negatively charged, with a strong affinity for calcium. SHMP can displace stain molecules from calcium binding sites. It binds to the tooth surface and integrates into the pellicle to prevent additional stain molecules from binding (Figure 22).48,49,81
Video 9. Formation of Extrinsic Stain.
b. Intrinsic stain. Intrinsic stains are stains and discolorations that are located below the enamel surface. Due to their diverse etiology, intrinsic stain treatment varies with the cause. Bleaching is usually used to remove or minimize intrinsic discolorations. The most popular bleaching agent is hydrogen peroxide (H2O2), in concentrations that range from 3% - 35%. Typically the higher concentration products are used for in-office bleaching, whereas products with 15% or lower concentrations are often used in home-applied whitening protocols (e.g., whitening strips).77,82,83 Whitening efficacy is dependent upon both hydrogen peroxide concentration and contact time with the dentition. Dentifrices typically do not remove intrinsic tooth stain because longer contact times are needed than what is achieved while brushing the teeth. Although some dentifrices or gels on the market for toothbrushing do contain hydrogen peroxide, concentration and contact time do not seem to be high enough to deliver intrinsic whitening. Rather, these products whiten teeth by the bleaching of surface stains.
Video 10. Removal of Extrinsic Stain.
Halitosis (Bad Breath). Halitosis, otherwise known as fetor oris, oral malodor or simply bad breath, is universally considered to be a socially unacceptable condition.84 Although prevalence estimates vary, it is clear that the condition affects a significant portion of the population, with an estimated 20-30% of adults reported to suffer from chronic breath malodor.84-90 Although a limited percentage of halitosis cases result from extraoral factors, such as diabetes, liver, kidney and other metabolic diseases,91 the highest percentage of cases are the result of intraoral causes,92 and are characterized by the production of gaseous volatile sulfur compounds (VSCs) associated with unpleasant bad breath.93,94 Hydrogen sulfide, methyl mercaptan and dimethyl sulfide are frequently cited as exhaled VSCs most commonly associated with unpleasant breath.92,95
Certain foods, especially ones like garlic and onions that contain pungent oils, can contribute to bad breath because the oils from these foods are eventually carried to your lungs and exhaled through your breath. Another source of bad breath can be individuals that experience ongoing sinus conditions, as sinus conditions can lead to a dry mouth. People with sinus conditions often have congested nasal passages and therefore need to breathe through their mouth. The drying effect of mouth breathing can create an environment that promotes bad breath. Additionally, sinus sufferers are likely to be taking antihistamines, a type of medicine that is known to create mouth dryness.
Video 11. Inhibition of Extrinsic Stain.
Even people who don't have an ongoing problem with bad breath easily notice that their breath is least pleasant in the morning when they first wake up. During the night, a person's salivary flow is reduced when a person sleeps. Saliva flow helps maintain mouth moisture, and it helps cleanse debris, bacteria and bacterial by-products that cause bad breath every time we swallow. As that effect is reduced overnight when we sleep, the result can be stale breath in the morning; a condition that is similarly noticed by people whose mouth becomes dry after speaking for long periods of time. Smoking is also considered to be a major cause of bad breath.
In the majority of cases, bad breath is caused by the presence of oral bacteria and oral debris.96 The bacteria and oral debris associated with breath malodor are largely found on the tongue as well as in subgingival and interproximal niches that are difficult to clean.97-99 In the absence of regular, thorough brushing and flossing, bacteria can accumulate on the bits of food left between the teeth, in the mouth and on the tongue. Sulfur compounds released by these bacteria give breath an unpleasant smell; with halitosis occurring when the unpleasant odor is then expelled from the mouth when exhaling. In addition to halitosis being an undesirable condition to have, it clearly has the potential to make social situations particularly unbearable.
Oral inflammatory diseases such as gingivitis and periodontitis are also associated with halitosis; a result of bacteria hiding in diseased tissues, producing foul gases.100,101 While meticulous oral hygiene in conjunction with scrupulous tongue brushing could theoretically help prevent persistent malodor, studies and surveys have shown that few adults regularly remove enough dental plaque through mechanical oral hygiene alone to alleviate the problem.102,103 In most cases, good professional oral care combined with a daily regimen of oral hygiene, including interdental cleaning, deep tongue cleaning and optional use of efficacious oral care products specially formulated to combat the germs that can cause bad breath, will lead to improvement.
Video 12. Formation of Volatile Sulfur Compounds (VSC) by Bacteria on Tongue.
a. Flavors to freshen breath. Bad breath sufferers often seek out help in the form of commercial products marketed to freshen objectionable breath. While some of these products are able to deliver a brief masking of the halitosis, most do not have the potential to provide long-term benefits. They are designed simply to temporarily mask odors. Unfortunately, many are quickly washed away by the natural flow of saliva. Methods used to help reduce bad breath, such as mints, mouth sprays, mouthwash or gum, may only temporarily mask the odors created by the bacteria on the tongue. These methods, however, cannot cure bad breath because they do not remove the source of the bad breath.
b. Antibacterial agents to reduce malodor. Antibacterial approaches can provide substantially longer-term breath efficacy than that provided by odor masking agents.104-106 As opposed to flavor agents that simply mask odor, antibacterial agents actually treat the source of the problem by targeting the malodor producing bacteria.
The use of zinc and triclosan are two dentifrice ingredients that have been identified for their ability to target bacteria and help reduce volatile compounds responsible for bad breath.107,108 Stannous fluoride, a well-studied antimicrobial agent with concurrent anticaries,68 desensitizing56,70and anti-plaque and gingivitis benefits47,69 has been found to be a particularly effective as a dentifrice ingredient compared to other approaches for its ability to provide both germ kill and breath protection.109
Video 13. Antibacterial Agent Reducing Bacteria and VSC.
Dentifrice Ingredients Providing Stability or Esthetic Benefits
This section will review key dentifrice ingredients that provide stability and esthetic benefits. These nontherapeutic dentifrice components are called inactive ingredients, additives, or excipients and include binders, surfactants, buffering agents, humectants, preservatives, sweeteners, flavorings, and dyes (Figure 23). These components are essential to keep the dentifrice properly mixed with a smooth consistency, and they make the product palatable to the consumer. Three dentifrice ingredients (abrasive, humectants, and solvent) typically represent about 95% of the dentifrice ingredients.
Humectants
Humectants retain moisture so that the dentifrice does not dry out. Humectants function by binding and holding the solvent in the dentifrice. Water is the solvent used in most dentifrices. Humectants, such as glycerin and sorbitol, also inhibit bacterial growth and provide flowability to the dentifrice. Humectants and solvent combined represent approximately 75% of a typical dentifrice formulation.
Binders
Binders, also referred to as thickeners, provide texture and determine how "thick" or "runny" the dentifrice is. Binders are used for cohesiveness, to provide body, and to prevent ingredients from separating. Xanthan gum, carboxymethyl cellulose (CMC) carbomers, carrageenan, and synthetic cellulose are all commonly used dentifrice binders. Binders are usually large polymeric polar molecules that form strong interactions with water. These interactions change the consistency and flowability of the dentifrice. Without the binder, the toothpaste would separate into different phases, a liquid portion and a solid-like portion, and would have to be stirred before each use.
Buffers
Manufacturers use buffers as part of their dentifrice formulation to keep the pH constant. This is important for the stability and effectiveness of a dentifrice. For example, pH can be an important factor in fluoride bioavailability; fluoride is more difficult to formulate at lower pH because of greater potential for interaction with common abrasives under acidic conditions. In some countries, regulations require dentifrice to be above a certain pH. Trisodium phosphate and sodium citrate are examples of dentifrice buffers. Pyrophosphates, which are used to control calculus formation, are also very effective buffers.
Flavors/Sweeteners
Flavoring agents and sweeteners are added to improve the dentifrice taste. This is a very important ingredient from a marketing standpoint, because consumers can have a strong preference for flavor. Most dentifrices have potent flavoring agents to mask the taste of some other ingredients that may have bitter or metallic tastes. Common flavoring agents and sweeteners include peppermint, saccharin, and xylitol.
Sweeteners used in dentifrices are all noncariogenic; and thus do not contribute to caries formation. Some dental professionals take special interest in knowing whether an oral care product contains xylitol because there are reports in the literature that it may provide a small anticaries benefit;110 however, xylitol is not approved by the US FDA as a proven anticaries agent. In the US, products utilize the FDA approved drug actives [sodium fluoride, sodium monofluorophosphate or stannous fluoride] to provide anticaries benefits.
Surfactants
Surfactants (detergents) create the foaming commonly associated with dentifrices. They aid in the cleaning process by helping to loosen plaque and debris. Sodium lauryl sulfate (SLS) is the surfactant most commonly used. SLS and other surfactants have been associated with the promotion of aphthous ulcers (canker sores) in a very small number of susceptible individuals. Surfactants also provide emulsion stability for the flavoring system. Without the surfactant in the toothpaste the flavor oil would not stay suspended in the product, causing oil separation from the product.
Colors/Visuals
Finally, coloring agents are added to provide dentifrice with pleasing colors. The opacity of a paste dentifrice comes from the addition of titanium dioxide. Dentifrices formulated without titanium dioxide result in the formation of a gel dentifrice, rather than an opaque paste. Mica is used to provide a sparkly appearance in some dentifrices, such as those marketed to children. A summary of dentifrice additives can be found in Table 5.
Compatibility Concerns
A primary concern when formulating a dentifrice is the need to prevent the inactivation of therapeutic agents by other ingredients. This is commonly the case with abrasive components (e.g., silica) that can bind to and limit the bioactivity of therapeutic ingredients. Other concerns involve the degradation or inactivation of ingredients by water. For example, SHMP can be hydrolyzed by water into individual phosphates or other intermediate breakdown products.
As noted throughout this course, a dentifrice is a very complex aggregate of chemicals with very specific functions. Not only do these ingredients have to be effective individually, they also have to be compatible with one another. All of these requirements demand very careful formulation and processing in order to be able to manufacture a high quality dentifrice.
Conclusion
The FDA uses two mechanisms to regulate OTC drugs: drug monographs and NDAs. A drug monograph identifies active ingredients that are deemed to be safe and effective for a specific therapeutic need. Most OTC fluoride-containing dentifrices are regulated through the Anticaries, Antiplaque-Antigingivitis, and Tooth Desensitizer monographs. If a dentifrice contains a drug that is not included in a monograph, it must be approved through an NDA. Therapeutic dentifrices brought to market under one of these two regulatory pathways can make claims related to treating or preventing disease.
The ADA is a professional society that takes great interest in informing the public on the safety and efficacy of oral care products. This is done primarily by awarding its Seal of Acceptance. The ADA Seal of Acceptance program is a rigorous, voluntary process in which manufacturers can choose to participate for specific products.
Fluoride was the first therapeutic ingredient used in dentifrice. Fluoride helps prevent caries by enhancing remineralization and inhibiting demineralization. The three fluoride ingredients approved by the FDA for use in dentifrices are stannous fluoride (SnF2), sodium fluoride (NaF), and sodium monofluorophosphate (Na2PO3F).
Since the introduction of early fluoride dentifrices, many other ingredients have been discovered and added to dentifrice to provide multiple additional benefits, including the following:
• Plaque/gingivitis/malodor reduction: Plaque, gingivitis, and halitosis are caused by bacteria. Antibacterial-containing dentifrices can help prevent these conditions. The exact mechanisms by which different agents exert antibacterial actions may differ.
• Antihypersensitivity: Dentinal hypersensitivity can be treated by chemically depolarizing nerve endings in the tooth or by blocking dentinal tubules. Potassium nitrate is the most common nerve desensitizing agent. Stannous fluoride, arginine + calcium carbonate, strontium acetate, and calcium sodium phosphosilicate are tubule occluding agents used in newer antihypersensitivity dentifrices across the globe.
• Calculus control: Polyphosphates, such as SHMP, are effective anticalculus agents. They chelate (bind) calcium and inhibit plaque calcification.
• Stain removal/Whitening: Stain removal or tooth whitening is achieved through chemical or physical action. Polyphosphates are good stain removal agents. They displace stain molecules that have attached to the tooth pellicle. Abrasives remove tooth stain through a physical action. Dentifrices with an RDA of 250 or lower are considered safe for everyday use.
Additional dentifrice ingredients include humectants, binders, buffers, flavors, sweeteners, and surfactants. These ingredients stabilize the product and create esthetic benefits for the consumer. They are needed to keep the dentifrice properly mixed with a palatable consistency. Not all dentifrice ingredients are compatible, however, so manufacturers must formulate products in a way that does not interfere with the bioavailability of the therapeutic ingredients. Creating a dentifrice that delivers important therapeutic and cosmetic benefits, while at the same time being acceptable to the consumer, requires the manufacturer to delicately balance the overall formulation.
Glossary
bioavailability - The degree to which a drug or substance is available to the target tissue following administration.
buffer - Chemical system that confers resistance to a change in the pH of a solution (e.g., saliva) when hydrogen ions (H+) are added or removed.
carbohydrate - Important energy source for the body; a complex molecule made up of one or more simple sugars.
calculus - Calcified plaque: a hard yellowish deposit on the teeth, consisting of organic secretions and food particles deposited in various salts, such as calcium carbonate; also called tartar.
caries - The process of dental decay, beginning with the earliest initiation of tooth demineralization and culminating with the collapse (cavitation) of a specific tooth surface. Dental caries is an infectious disease caused by the complex interaction of certain plaque bacteria with carbohydrates (i.e., sugars), resulting in the generation of acids that can attack and damage both enamel and dentin.
cariogenic - Contributing to the production of caries.
chelate - Action of certain chemical compounds whereby they form several noncovalent bonds to a single metal ion (e.g., Ca2+), sequestering it and preventing it from reacting with its surroundings.
chromogen - Substance that can be converted to a pigment or dye.
compound - In chemistry, a substance that consists of two or more chemical elements in union.
covalent - In chemistry, a chemical bond formed by the sharing of one or more electrons, especially pairs of electrons, between atoms.
crevicular - A fluid produced by epithelium of the gingival crevice; it contains immunoglobulins and has antimicrobial properties.
dental erosion - Irreversible loss of tooth structure resulting from strong acids of nonbacterial origin (e.g. dietary, gastric).
enzyme - Protein that catalyzes, or facilitates, biochemical reactions.
extrinsic stain - Tooth stain on the exterior surface of the tooth that can be removed through routine cleaning procedures. It is generally composed of dietary chromogenic molecules and metal ions which become bound within the salivary pellicle layer that coats exposed tooth surfaces.
gingivitis - Inflammation of the gums that often manifests as bleeding during brushing and flossing; mildest form of periodontal disease that is reversible.
heme - A complex red organic pigment containing iron and other atoms to which oxygen binds.
halitosis - The condition of having stale or foul-smelling breath.
hydrophobic - Water-resisting; refers to a chemical entity that repels water and prefers oily environments.
ions - Atoms or molecules that carry either a positive or a negative electric charge in a solution. For example, sodium chloride (NaCl, common table salt) in water dissociates into Na+ and Cl- ions.
intrinsic stain - Staining caused by the presence of pigment within the enamel or dentine. Intrinsic stain can often be mediated through bleaching procedures.
lysis - The destruction or dissolution of a cell or molecule, generally through the action of a specific agent.
metabolize - The process through which food is broken down to release energy.
molecule - Chemical entity that consists of two or more atoms that have chemically combined to form a single species.
New drug application (NDA) - Application requesting FDA approval to market a new drug, drug formulation, or dose.
noncavitated lesion - Demineralized, subsurface carious lesion without evidence of discontinuity or break in the enamel surface (sometimes called an early lesion, incipient lesion, or white spot lesion).
organic acids - Acid containing at least one carbon atom; also called a carboxylic acid; written chemically as: see Figure 24
over-the-counter (OTC) - Drug products that are generally recognized as safe and effective and are available without a prescription; in oral care, many dentifrices and some rinses are OTC products.
OTC Monograph - A document published by the US FDA that includes lists of ingredients that have proven effectiveness and safety for a particular health concern, as well as information about dosing, drug formulations and labeling.
patency - State or quality of being open, expanded, or unblocked.
pharmacology - Study of a drug's origin, chemistry, effects, and uses.
plaque - An organized community of many different microorganisms that forms itself into a biofilm and is found on the surface of the tongue and all hard surfaces in the oral cavity. Dental plaque is present in all people and can vary from being comprised of totally healthy microorganisms (commensals) to being very harmful (pathogenic), predisposing the patient to dental caries or periodontal diseases. Note: Dental plaque is not food debris, nor does it contain food debris. Dental plaque can only be completely removed by mechanical means, such as toothbrushing or prophylaxis.
subgingival - Located beneath the free margin of gingival tissue.
supragingival - Located on a portion of the tooth that is not surrounded by gingival tissue.
surfactant - Compounds such as detergents, emulsifiers, and foaming agents that provide cleaning or help mix substances that prefer to separate (like oil and water). Surfactants typically have a hydrophilic, polar head that interacts with water and a hydrophobic, nonpolar tail that avoids water.
tartar - Calcified plaque: a hard yellowish deposit on the teeth, consisting of organic secretions and food particles deposited in various salts, such as calcium carbonate; also called calculus.
toxicology - Study of the unwanted and often adverse effects of substances.
References
1. US Federal Food, Drug, & Cosmetic Act (FFDCA). Accessed June 5, 2017.
2. Tenuta LM, Cury JA. Fluoride: its role in dentistry. Braz Oral Res. 2010;24 Suppl 1:9-17.
3. US FDA Monograph System. Accessed June 5, 2017.
4. US Food & Drug Administration (FDA). Accessed June 5, 2017.
5. American Dental Association (ADA). Seal of Acceptance Program. Accessed June 5, 2017.
6. American Dental Association (ADA). Seal Program Guidelines. Accessed June 5, 2017.
7. Ten Great Public Health Achievements - United States, 1900-1999, CDC Morbidity and Mortality Weekly Report 1999; 48 (12) 241-243. Accessed June 5, 2017.
8. Bratthall D, Hänsel-Petersson G, Sundberg H. Reasons for the caries decline: what do the experts believe? Eur J Oral Sci. 1996 Aug;104(4 ( Pt 2)):416-22; discussion 423-5, 430-2.
9. Tenuta LM, Zamataro CB, Del Bel Cury AA, et al. Mechanism of fluoride dentifrice effect on enamel demineralization. Caries Res. 2009;43(4):278-85. doi: 10.1159/000217860. Epub 2009 May 8.
10. Cury JA, do Amaral RC, Tenuta LM, et al. Low-fluoride toothpaste and deciduous enamel demineralization under biofilm accumulation and sucrose exposure. Eur J Oral Sci. 2010 Aug;118(4):370-5. doi: 10.1111/j.1600-0722.2010.00745.x.
11. Muhler JC, Radike AW, Nebergall WH, et al. The effect of a stannous fluoride-containing dentifrice on caries reduction in children. J Dent Res. 1954 Oct;33(5):606-12. doi: 10.1177/00220345540330050401.
12. Muhler JC, Radike AW. Effect of a dentifrice containing stannous fluoride on dental caries in adults. II. Results at the end of two years of unsupervised use. J Am Dent Assoc. 1957 Aug;55(2):196-8.
13. American Dental Association, Council on Dental Therapeutics: Evaluation of Crest toothpaste. JADA 1960; 61:272-274.
14. Chace R, American Dental Association. Reclassification of Crest toothpaste; Council on Dental Therapeutics. J Am Dent Assoc. 1964 Aug;69:195-6.
15. Chronology of important chemical and related events since 1876. Chemical and Engineering News April 6th, 1976: 91-92.
16. Featherstone JD. The caries balance: the basis for caries management by risk assessment. Oral Health Prev Dent. 2004;2 Suppl 1:259-64.
17. Fejerskov O. Concepts of dental caries and their consequences for understanding the disease. Community Dent Oral Epidemiol. 1997 Feb;25(1):5-12.
18. Silverstone LM. Laboratory studies on the demineralization and remineralization of human enamel in relation to caries mechanisms. Aust Dent J. 1980 Jun;25(3):163-8.
19. Cury JA, Tenuta LM. Enamel remineralization: controlling the caries disease or treating early caries lesions? Braz Oral Res. 2009;23 Suppl 1:23-30.
20. Posner AS. The mineral of bone. Clin Orthop Relat Res. 1985 Nov;(200):87-99.
21. Dijkman A, Huizinga E, Ruben J, et al. Remineralization of human enamel in situ after 3 months: the effect of not brushing versus the effect of an F dentifrice and an F-free dentifrice. Caries Res. 1990;24(4):263-6.
22. Clarkson JE, Ellwood RP, Chandler RE. A comprehensive summary of fluoride dentifrice caries clinical trials. Am J Dent. 1993 Sep;6 Spec No:S59-106.
23. White DJ. A "return" to stannous fluoride dentifrices. J Clin Dent. 1995;6 (Spec Iss):29-36.
24. Baig A, He T. A novel dentifrice technology for advanced oral health protection: A review of technical and clinical data. Compend Contin Educ Dent. 2005 Sep;26(9 Suppl 1):4-11.
25. Bibby BG. Fluoride mouthwashes, fluoride dentifrices, and other uses of fluorides in control of caries. J Dent Res. 1948 Jun;27(3):367-75. doi: 10.1177/00220345480270031301.
26. Stookey GK. Are all fluoride dentifrices the same? In: Wei SHY Clinical uses of fluoride. Lea & Febiger 1985, Philadelphia.
27. Zacherl WA. A three-year clinical caries evaluation of the effect of a sodium fluoride-silica abrasive dentifrice. Pharmacol Ther Dent. 1981;6(1-2):1-7.
28. Beiswanger BB, Gish CW, Mallatt ME. A three-year study of the effect of a sodium fluoride-silica abrasive dentifrice on dental caries. Pharmacol Ther Dent. 1981;6(1-2):9-16.
29. Colgate Cavity Protection Information. Colgate-Cavity-Protection-Toothpaste/specifics. Accessed June 5, 2017.
30. Shellis RP, Duckworth RM. Studies on the cariostatic mechanisms of fluoride. Int Dent J. 1994 Jun;44(3 Suppl 1):263-73.
31. American Dental Association. Joint Report of the American Dental Association Council on Access, Prevention and Interprofessional Relations and Council on Scientific Affairs to the House of Delegates: Response to Resolution 73H-2000. October 2001. Accessed June 5, 2017.
32. Imfeld T. Dental erosion. Definition, classification and links. Eur J Oral Sci. 1996 Apr;104(2 (Pt 2)):151-5.
33. ten Cate JM, Imfeld T. Dental erosion, summary. Eur J Oral Sci. 1996 Apr;104(2 ( Pt 2)):241-4.
34. Johansson AK, Lingström P, Imfeld T, et al. Influence of drinking method on tooth-surface pH in relation to dental erosion. Eur J Oral Sci. 2004 Dec;112(6):484-9. doi: 10.1111/j.1600-0722.2004.00172.x.
35. Hooper SM, Newcombe RG, Faller R, et al. The protective effects of toothpaste against erosion by orange juice: studies in situ and in vitro. J Dent. 2007; 35(6): 476-481. doi: 10.1016/j.jdent.2007.01.003.
36. Huysmans MCDNJM, Jager DHJ, Ruben JL, et al. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011; 45:518-523. doi: 10.1159/000331391.
37. Bellamy P, Harris R, Date R, et al. In situ clinical evaluation of a stabilised, stannous fluoride dentifrice. Int Dent J 2014; 64(Suppl 1): 43-60. doi: 10.1111/idj.12102.
38. Faller RV, Eversole SL. Enamel protection from acid challenge - benefits of marketed fluoride dentifrices. J Clin Dent 2013; 24(1):25-30.
39. Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion protection comparison of stabilised SnF2, mixed fluoride active and SMFP/arginine-containing dentifrices. Int Dent J 2014; 64(Suppl 1):22-28. doi: 10.1111/idj.12099.
40. Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion prevention potential of an over-the-counter stabilized SnF2 dentifrice compared to 5000ppm F prescription strength products. J Clin Dent 2015; 2015;26:44-49.
41. Faller RV, Eversole SL, Tzeghai GE. Enamel protection: a comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011 Aug;24(4):205-10.
42. Baig AA, Faller RV, Yan J, et al. Protective effects of SnF2 - Part 1. Mineral solubilisation studies on powdered apatite. Int Dent J 2014;64 (Suppl 1):4-10. doi: 10.1111/idj.12096.
43. Khambe D, Eversole SL, Mills T, et al. Protective effects of SnF2 - Part II. Deposition and retention on pellicle-coated enamel. Int Dent J. 2014 Mar;64 Suppl 1:11-5. doi: 10.1111/idj.12097.
44. Faller RV, Eversole SL. Protective effects of SnF2 - Part III, Mechanism of barrier layer attachment. Int Dent J. 2014 Mar;64 Suppl 1:16-21. doi: 10.1111/idj.12098.
45. Lussi A, Carvalho TS. The future of fluorides and other protective agents in erosion prevention. Caries Res. 2015;49 Suppl 1:18-29. doi: 10.1159/000380886. Epub 2015 Apr 13.
46. Ramji N, Baig A, He T, Lawless MA, et al. Sustained antibacterial actions of a new stabilized stannous fluoride dentifrice containing sodium hexametaphosphate. Compend Contin Educ Dent. 2005 Sep;26(9 Suppl 1):19-28.
47. Mankodi S, Bartizek RD, Winston JL, et al. Anti-gingivitis efficacy of a stabilized 0.454% stannous fluoride/sodium hexametaphosphate dentifrice. J Clin Periodontol. 2005 Jan;32(1):75-80. doi: 10.1111/j.1600-051X.2004.00639.x.
48. Baig A, He T, Buisson J, et al. Extrinsic whitening effects of sodium hexametaphosphate-a review including a dentifrice with stabilized stannous fluoride. Compend Contin Educ Dent. 2005 Sep;26(9 Suppl 1):47-53.
49. Terézhalmy G, Chaves E, Bsoul S, et al. Clinical evaluation of the stain removal efficacy of a novel stannous fluoride and sodium hexametaphosphate dentifrice. Am J Dent. 2007 Feb;20(1):53-58.
50. Ledder RG, Sreenivasan PK, DeVizio W, et al. Evaluation of the specificity and effectiveness of selected oral hygiene actives in salivary biofilm microcosms. J Med Microbiol. 2010 Dec;59(Pt 12):1462-1468. doi: 10.1099/jmm.0.024372-0.
51. Davies RM, Ellwood RP, Davies GM. The effectiveness of a toothpaste containing triclosan and polyvinyl-methyl ether maleic acid copolymer in improving plaque control and gingival health: a systematic review. J Clin Periodontol. 2004 Dec;31(12):1029-1033. doi: 10.1111/j.1600-051X.2004.00614.x.
52. Davies RM. The clinical efficacy of triclosan/copolymer and other common therapeutic approaches to periodontal health. Clin Microbiol Infect. 2007 Oct;13 Suppl 4:25-29. doi: 10.1111/j.1469-0691.2007.01801.x.
53. Deasy MJ, Singh SM, Rustogi KN, et al. Effect of a dentifrice containing triclosan and a copolymer on plaque formation and gingivitis. Clin Prev Dent. 1991 Nov-Dec;13(6):12-19.
54. Bolden TE, Zambon JJ, Sowinski J, et al. The clinical effect of a dentifrice containing triclosan and a copolymer in a sodium fluoride/silica base on plaque formation and gingivitis: a six-month clinical study. J Clin Dent. 1992;3(4):125-131.
55. Feller RP, Kiger RD, Triol CW, et al. Comparison of the clinical anticaries efficacy of an 1100 NaF silica-based dentifrice containing triclosan and a copolymer to an 1100 NaF silica-based dentifrice without those additional agents: a study on adults in California. J Clin Dent. 1996;7(4):85-89.
56. Schiff T, He T, Sagel L, et al. Efficacy and safety of a novel stabilized stannous fluoride and sodium hexametaphosphate dentifrice for dentinal hypersensitivity. J Contemp Dent Pract. 2006 May 1;7(2):1-8.
57. Johnson G, Brännström M. The sensitivity of dentin. Changes in relation to conditions at exposed tubule apertures. Acta Odontol Scand. 1974;32(1):29-38.
58. Närhi MV. Dentin sensitivity: a review. J Biol Buccale. 1985 Jun;13(2):75-96.
59. Cox CF. Etiology and treatment of root hypersensitivity. Am J Dent. 1994 Oct;7(5):266-270.
60. Holland GR, Narhi MN, Addy M, et al. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997 Nov;24(11):808-813.
61. Irwin CR, McCusker P. Prevalence of dentine hypersensitivity in a general dental population. J Ir Dent Assoc. 1997;43(1):7-9.
62. Marieb EN, Hoehn K. Human anatomy and physiology. 7th ed. San Francisco CA: Pearson Benjamin Cummings. 2007.
63. Brännström M, Johnson G, Nordenvall KJ. Transmission and control of dentinal pain: resin impregnation for the desensitization of dentin. J Am Dent Assoc. 1979 Oct;99(4):612-618.
64. Petrou I, Heu R, Stranick M, et al. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20(1):23-31.
65. Litkowski L, Greenspan DC. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity--proof of principle. J Clin Dent. 2010;21(3):77-81.
66. Gendreau L, Barlow AP, Mason SC. Overview of the clinical evidence for the use of NovaMin in providing relief from the pain of dentin hypersensitivity. J Clin Dent. 2011;22(3):90-95.
67. White DJ, Lawless MA, Fatade A, et al. Stannous fluoride/sodium hexametaphosphate dentifrice increases dentin resistance to tubule exposure in vitro. J Clin Dent. 2007;18(2):55-59.
68. Stookey GK, Mau MS, Isaacs RL, et al. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004 Nov-Dec;38(6):542-550. doi: 10.1159/000080584.
69. Mallatt M, Mankodi S, Bauroth K, et al. A controlled 6-month clinical trial to study the effects of a stannous fluoride dentifrice on gingivitis. J Clin Periodontol. 2007 Sep;34(9):762-767. doi: 10.1111/j.1600-051X.2007.01109.x.
70. Schiff T, Saletta L, Baker RA, et al. Desensitizing effect of a stabilized stannous fluoride/Sodium hexametaphosphate dentifrice. Compend Contin Educ Dent. 2005 Sep;26(9 Suppl 1):35-40.
71. Jin Y, Yip HK. Supragingival calculus: formation and control. Crit Rev Oral Biol Med. 2002;13(5):426-441.
72. Bollmer BW, Sturzenberger OP, Vick V, et al. Reduction of calculus and Peridex stain with Tartar-Control Crest. J Clin Dent. 1995;6(4):185-187.
73. Pradeep AR, Agarwal E, P AR, et al. Study of orthophosphate, pyrophosphate, and pyrophosphatase in saliva with reference to calculus formation and inhibition. J Periodontol. 2011 Mar;82(3):445-451. doi: 10.1902/jop.2010.100355.
74. White DJ. A new and improved "dual action" whitening dentifrice technology-sodium hexametaphosphate. J Clin Dent. 2002;13(1):1-5.
75. White D, Cox E, Suszcynskymeister E, et al. In vitro studies of the anticalculus efficacy of a sodium hexametaphosphate whitening dentifrice. J Clin Dent. 2002;13(1):33-37.
76. Segreto VA, Collins EM, D'Agostino R, et al. Anticalculus effect of a dentifrice containing 0.5% zinc citrate trihydrate. Community Dent Oral Epidemiol. 1991 Feb;19(1):29-31.
77. Hattab FN, Qudeimat MA, al-Rimawi HS. Dental discoloration: an overview. J Esthet Dent. 1999;11(6):291-310.
78. Schemehorn BR, Moore MH, Putt MS. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J Clin Dent. 2011;22(1):11-18.
79. Hefferren JJ. A laboratory method for assessment of dentifrice abrasivity. J Dent Res. 1976 Jul-Aug;55(4):563-573. doi: 10.1177/00220345760550040301.
80. St John S, White DJ. History of the Development of Abrasivity Limits for Dentifrices. J Clin Dent. 2015;26(2):50-4.
81. He T, Baker R, Bartizek RD, et al. Extrinsic stain removal efficacy of a stannous fluoride dentifrice with sodium hexametaphosphate. J Clin Dent. 2007;18(1):7-11.
82. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989 Mar;20(3):173-176.
83. Dorfman WM. News from Procter and Gamble (P&G) introducing Crest Whitestrips, their new over-the-counter whitening product. J N J Dent Assoc. 2000 Fall;71(4):7, 10.
84. Feng X, Chen X, Cheng R, et al. Breath malodor reduction with use of a stannous-containing sodium fluoride dentifrice: a meta-analysis of four randomized and controlled clinical trials. Am J Dent. 2010 Sep;23 Spec No B:27B-31B.
85. Bosy A. Oral malodor: philosophical and practical aspects. J Can Dent Assoc. 1997 Mar;63(3):196-201.
86. Liu XN, Shinada K, Chen XC, et al. Oral malodor-related parameters in the Chinese general population. J Clin Periodontol. 2006 Jan;33(1):31-36. doi: 10.1111/j.1600-051X.2005.00862.x.
87. Miyazaki H, Sakao S, Katoh Y, et al. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J Periodontol. 1995 Aug;66(8):679-684. doi: 10.1902/jop.1995.66.8.679.
88. Ayo-Yusuf OA, Van Wyk C, Van Wyk P. Oral malodour among patients attending a preventive clinic in Pretoria. J Dent Res 2005;84 Sp Iss B).
89. Iwanicka-Grzegorek E, Michalik J, Kepa J, et al. Subjective patients' opinion and evaluation of halitosis using halimeter and organoleptic scores. Oral Dis. 2005;11 Suppl 1:86-88. doi: 10.1111/j.1601-0825.2005.01101.x.
90. Bornstein MM, Kislig K, Hoti BB, et al. Prevalence of halitosis in the population of the city of Bern, Switzerland: a study comparing self-reported and clinical data. Eur J Oral Sci. 2009 Jun;117(3):261-267. doi: 10.1111/j.1600-0722.2009.00630.x.
91. Preti G, Clark L, Cowart BJ, et al. Non-oral etiologies of oral malodor and altered chemosensation. J Periodontol. 1992 Sep;63(9):790-796. doi: 10.1902/jop.1992.63.9.790.
92. ADA Council on Scientific Affairs. Oral malodor. J Am Dent Assoc. 2003 Feb;134(2):209-214.
93. Kleinberg I, Westbay G. Salivary and metabolic factors involved in oral malodor formation. J Periodontol. 1992 Sep;63(9):768-775. doi: 10.1902/jop.1992.63.9.768.
94. Rosenberg M. The science of bad breath. Sci Am. 2002 Apr;286(4):72-79.
95. Rosenberg M. Bad breath: Research perspectives. Ramat Aviv: Ramot Publishing-Tel Aviv University 1997.
96. Haraszthy VI, Zambon JJ, Sreenivasan PK, et al. Identification of oral bacterial species associated with halitosis. J Am Dent Assoc. 2007 Aug;138(8):1113-1120.
97. De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. J Am Dent Assoc. 1995 Oct;126(10):1384-1393.
98. Rosenberg M. Clinical assessment of bad breath: current concepts. J Am Dent Assoc. 1996 Apr;127(4):475-482.
99. Lee CH, Kho HS, Chung SC, et al. The relationship between volatile sulfur compounds and major halitosis-inducing factors. J Periodontol. 2003 Jan;74(1):32-37. doi: 10.1902/jop.2003.74.1.32.
100. Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992 Jul;27(4 Pt 1):233-238.
101. Ratcliff PA, Johnson PW. The relationship between oral malodor, gingivitis, and periodontitis. A review. J Periodontol. 1999 May;70(5):485-489. doi: 10.1902/jop.1999.70.5.485.
102. Bakdash B. Current patterns of oral hygiene product use and practices. Periodontol 2000. 1995 Jun;8:11-14.
103. van der Weijden GA, Hioe KP. A systematic review of the effectiveness of self-performed mechanical plaque removal in adults with gingivitis using a manual toothbrush. J Clin Periodontol. 2005;32 Suppl 6:214-228. doi: 10.1111/j.1600-051X.2005.00795.x.
104. Farrell S, Barker ML, Gerlach RW. Overnight malodor effect with a 0.454% stabilized stannous fluoride sodium hexametaphosphate dentifrice. Compend Contin Educ Dent. 2007 Dec;28(12):658-661.
105. Tinanoff N. Progress regarding the use of stannous fluoride in clinical dentistry. J Clin Dent. 1995;6 Spec No:37-40.
106. Donovan-Brand R. A comparison of the breath and antimicrobial efficacies of various antimicrobial systems present in commercial dental products. J Dent Res 2003; 82 (Sp Iss):501.
107. Navada R, Kumari H, Le S, et al. Oral malodor reduction from a zinc-containing toothpaste. J Clin Dent. 2008;19(2):69-73.
108. Hu D, Zhang YP, Petrone M, et al. Clinical effectiveness of a triclosan/copolymer/sodiumfluoride dentifrice in controlling oral malodor: a three-week clinical trial. Compend Contin Educ Dent. 2003 Sep;24(9 Suppl):34-41.
109. Chen X, He T, Sun L, et al. A randomized cross-over clinical trial to evaluate the effect of a 0.454% stannous fluoride dentifrice on the reduction of oral malodor. Am J Dent. 2010 Jun;23(3):175-178.
110. Mäkinen KK. Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med Princ Pract. 2011;20(4):303-320. doi: 10.1159/000324534.
About the Authors
Paula M. Koenigs, PhD
The P&G community is saddened by the loss of our friend and colleague, Dr. Paula Koenigs, on November 28, 2016. She was an outstanding scientist who touched many lives around the world with her passion for research and training. We will miss her.
Dr. Koenigs was a Principal Scientist at The Procter & Gamble Company. Dr. Koenigs attended the University of Kansas as a Watkins-Berger Scholar where she received a Bachelor of Science in Chemistry and was awarded the Clark E. Bricker Scholarship for Chemistry. She attended Duke University to receive her doctorate in Physical Organic Chemistry where she researched the inhibition and photo-reactivation of serine protease enzymes in the blood coagulation cascade. While at Duke University she was the recipient of an NIH Pharmacology Training Grant. Dr. Koenigs worked for P&G for 23 years. Her first 10 years at P&G were spent in drug discovery research with P&G Pharmaceuticals in the area of bacterial infectious disease. Since that time she was involved with technical communication and training in the areas of osteoporosis, respiratory sciences and oral care.
Robert V. Faller, BS
Robert Faller retired from P&G after more than 31 years in the Oral Care Research field, where he focused on caries and enamel related research as P&G's chief cariologist. He is currently a Clinical Associate Professor in Temple University's Maurice H. Kornberg School of Dentistry. He is editor of Volume 17 - Monographs in Oral Science: Assessment of Oral Health - Diagnostic Techniques and Validation Criteria, and has over 130 publications and published abstracts on fluoride, caries, dental erosion, and various oral care technologies, along with 5 patents related to Oral Care.
Email: robert.faller@yourencore.com