You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Saliva plays a well-defined role in the maintenance of health, comfort, esthetics, and function.1 Saliva lubricates; it has serous and mucinous components that allow the unrestricted movement of mucosa, soft tissue against hard tissue, tongue against cheek, teeth, and chewing. Saliva buffers against the decrease in pH that happens during mastication and on introduction of acidic drinks. Further, saliva encourages the ability to taste different flavors of foods, and it flushes the oral cavity, maintaining large debris and other items to be swallowed or to exit the oral cavity. Saliva also aids in bolus formation before swallowing and after food has been chewed, and it contains enzymes such as amylase and lipase that initiate the digestion of carbohydrates and fat. It helps remineralize the surface of teeth, and it contains the antimicrobial component lactoferrin, which sequesters iron to diminish the availability of iron for microbes, as well as peroxidase.1
Typically, salivary dysfunction presents as a complaint of xerostomia, which may or may not be indicative of actual salivary gland hypofunction.2 Xerostomia is commonly called “dry mouth”; it is the subjective sensation that patients are referring to in telling a healthcare provider that they have a dry mouth or decreased saliva.2-4 Salivary gland hypofunction, meanwhile, is defined as diminished salivary flow rate, typically accepted as a 50% decrease in the clinically determined rate in a healthy individual not taking medication.4 The resting flow rate occurs for the majority of the day, between 0.3 and 0.4 ml per minute.2 This rate is considered as the basal rate, important for oral comfort and protection throughout the day. There is also a stimulated flow rate, which is between 1 and 2 ml per minute.2 This rate represents the functional capacity of the functioning gland and is important for swallowing and oral clearing, particularly during functions such as eating, and occurs for about 2 hours over the course of the day.1,2
The challenge for oral healthcare practitioners is recognizing that there are objective and subjective complaints and then determining whether there is a deficiency of saliva in a patient. The most commonly encountered clinical signs and symptoms of xerostomia are3,5:
• persistent need for fluids
• dryness of mucous membranes
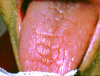
• tongue fissuring and lobulation
• angular cheilosis and cheilitis
• fungal infections
• amputation caries
• thick ropy saliva (sometimes filmy)
• dysphagia
• dysgeusia
• difficulty eating or speaking
• smacking lips together
• challenges wearing a prosthesis
• swelling of the salivary glands
• difficulty expressing saliva
• cheek biting
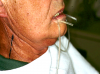
Asking a variety of questions related to these signs can help inform the diagnosis.2 Additionally, during the clinical examination, the buccal mucosa should be checked to see whether it sticks to gloves and mirrors,2 along with examining the tongue for fissuring (Figure 1) and measuring major gland secretions using a resting flow rate with a Carlson-Crittenden cup (Figure 2) or measuring stimulated flow rates using citric acid or paraffin wax to chew on.2,6 Minor gland secretions can be measured using an absorbent paper strip and doing volumetric analysis.2
Causes of Dry Mouth
Aging-related factors, age-related hormonal changes, disease, systemic factors, and medications can all contribute to salivary gland hypofunction. Additional sources can include environmental insults and trauma, such as head-and-neck radiation therapy and chemotherapy. Insights into the various etiologies are described below.
Aging
In and of itself, aging in people who are healthy and not taking medications is not a source of significant change in salivary quantity or quality. However, salivary gland hypofunction is associated with decreases or challenges in health that lead to taking multiple medications, and the population older than 65 years accounts for most prescribed and over-the-counter medications.2,7-10
Menopause
The average age of onset of menopause in the United States is 51 years.11 Although oral symptoms are common, cross-sectional and longitudinal studies have failed to provide significant or reproducible evidence that salivary flow is affected.12 As with the association to aging, these oral complaints are most likely the result of the xerostomic medications taken because of systemic complaints associated with menopause, such as antihypertensives, antidepressants, and antihistamines.2,12
Systemic Disease
Systemic diseases such as Sjögren syndrome or diabetes, as well as forms of dementia and neurological disorders, particularly Alzheimer’s disease, can be associated with xerostomia.2,13-23 Sjögren syndrome is an autoimmune disorder affecting the lacrimal and salivary glands.19-23 Keratoconjunctivitis sicca and xerostomia are the common complaints.21 The disease can be primary or secondary. The primary disease is when Sjögren occurs alone, with no other autoimmune disorder; it is secondary if it is associated with another autoimmune disease, such as rheumatoid arthritis, systemic lupus erythematosus, or multiple sclerosis.19,22,23 Histologically, the syndrome is characterized by dense inflammatory infiltrates with destruction of glandular tissue, and because there is destruction, the treatment is palliative. Currently there is no way to reverse the effects on the salivary tissue.20
In patients with diabetes, uncontrolled blood glucose levels may contribute to xerostomia.17,18 Medications that are used to manage diabetes may induce xerostomia; there may be enlargement and inflammation of the parotid glands, which is generally common in endocrine disorders, leading to complaints of dry mouth and difficulty in warding off infection, as well contributing to fungal infection such as candidiasis, gingivitis, periodontal disease, and caries, all of which can contribute to dry mouth.17,18
Alzheimer’s disease may affect the neurological components of salivary production and flow, as could other neurodegenerative disorders.13-16 Medications that are used to manage Alzheimer’s disease can contribute to xerostomia, and the disease-associated cognitive decline can complicate behavior, making it difficult to maintain a healthy dentition. Poor cooperation with dental care and treatment in a conventional setting as well as a deficiency in oral hygiene from the patient can be complicated by an inability to cooperate to have others help.13-16
Dehydration
Dehydration can come from a variety of different sources: for example, sweating in the summer, diarrhea, emesis, or blood loss. Symptoms in severe cases include flushed face, dry warm skin, fatigue, cramping, and reduced amount of urine. Oral signs and symptoms include dry mouth, dry tongue, thick sticky saliva, and dry cracked lips as well as dryness and cracking in the corners of the lip.24 It is important to determine whether these symptoms are a result of dehydration from a pathologic condition or just from not drinking enough water, perhaps on a hot day or during an increased amount of physical activity.
Head-and-Neck Cancer and Its Associated Treatment
Special cases apply to xerostomia or dry mouth related to cancer chemotherapy or head-and-neck radiation therapy, where salivary gland destruction in the path of radiation can be prominent.25-30 In external beam radiation therapy, where the radiation intensity is modulated dependent on the tissues that are involved, there is an attempt to minimize the collateral damage to structures such as the salivary glands, when they themselves are not the intended target, because the salivary gland tissue and mucosa are particularly susceptible to the permanent effects of radiation.25-30
When patients are seen prior to radiation therapy, an aggressive approach is recommended to reduce post-radiation morbidity. Any sign of infection or pathology needs to be treated in a definitive way. Teeth with moderate or advanced periodontal disease and teeth with endodontic pathology must be extracted to prevent poor outcomes when they are in the path of the radiation.31 Also, after any kind of invasive therapy, such as extractions, there should be a 10- to 14-day waiting period before initiating radiation.31 Because the destruction is permanent, aggressive prevention during and after radiation therapy is very important; the tissue that is affected by the radiation will remain hypocellular, hypovascular, and hypoxic, making it challenging to heal.27,29
In contrast, during most chemotherapy, the salivary glands are affected, but they will rebound after therapy.32 As with radiation therapy, aggressive dentistry is required before chemotherapy, and the time of chemotherapy should be the focus of aggressive prevention in the context of the patient’s immunosuppression. That is when the patient is most at risk for adverse effects on the salivary gland and on the immune system. After a patient has chemotherapy and is allowed to recover from the drugs, the patient’s salivary gland function is expected to return to pretreatment levels, or very near to that.32
Physical Obstructions
Tumors or other physical obstructions in the salivary glands and ducts as well as infections—bacterial, viral, and secondary to other diseases—can all contribute to xerostomia.33,34 One common condition is a sialolithiasis, which is a stone or a little calculus formation in the duct, physically blocking the egress of saliva.33,34 It happens mostly in the submandibular gland; symptoms include painful swelling that increases at mealtime. Its physical detection can be achieved by manual palpation in the floor of the mouth or through radiography and imaging. Typically, management involves analgesics and trying to push the stone out; however, there may be a dilation or surgical procedure needed to remove the stone, and if a superimposed bacterial infection is suspected, antibiotics may be needed.33,34
Inflammation and infection from bacterial sources can also lead to swelling and obstruction of the duct, known as sialadenitis. This occurs frequently in the parotid ducts.35 Chronic recurrent sialadenitis may be secondary to a physical obstruction or unusual anatomy or perhaps even a resistant organism; treatment includes antibiotics, hydration, stimulation of saliva, and, in rare instances, open drainage or some type of surgery.35
Medications
Some medication classes known to contribute to salivary gland hypofunction are listed below.36-47
• anticholinergics
• antihistamines
• antidepressants
• antipsychotics
• sedatives and hypnotic agents
• antihypertension agents
• anti-Parkinson agents
Although there is a reported increased effect with prolonged use of drugs and multiple drugs, it is most often seen in elderly patients, where there is an increased use of drugs.46 A few clinical trials and studies have definitively established this relationship. Researchers are uncertain what causes or facilitates the salivary gland hypofunction, but there are three main theories: interference with neural transmission through the parasympathetic nerves, dehydration, and vasoconstriction in salivary glands, which can change the flow and composition of saliva.48 If it is concluded or suspected that the xerostomia is medication-induced, then the prescribing physician should be consulted to see whether there is way to make modifications to help improve the patient’s situation.2,49,50
Managing Salivary Dysfunction
The management of salivary dysfunction includes encouraging patients to visit the dentist regularly, addressing problems when they first appear, and encouraging meticulous oral hygiene; the dry mouth places the patient at a very high caries risk because of the decreased flushing, decreased buffering, and decreased antimicrobial agent. An aggressive prevention protocol is recommended.51 The patient should be encouraged to stay well-nourished and well-hydrated, with an explanation of why this is necessary. An updated list of all medications the patient is taking should be kept: medications taken regularly as well as those taken only as needed. Medical, lifestyle/behavioral, and dietary histories should be updated often, and the dental professional should keep in communication with the patient’s physicians and other healthcare providers, consulting them when needed.51
As noted above, oral hygiene can be the key to good management. It is not always convenient to brush or rinse with a fluoride rinse; however, rinsing with water after eating, or even wiping down with a little piece of gauze is very helpful to remove loose debris. Removable prostheses should be rinsed and wiped out using a denture brush and an antifungal soap if there is an incident of fungal infection or candidiasis. Removing the prosthesis at night or even between meals when one is in the comfort of one’s own home may also reduce irritation/discomfort associated with dry mouth. Mechanical plaque removal for people with dry mouth includes soft toothbrushes and moist gauze. Oral swabs are good for soft-tissue cleaning for edentulous patients. A mild toothpaste should be used and alcohol-containing products avoided.2 Interdental aids will help as well. A variety of different products, including plaque removers, proxy brushes, different types of floss, and floss holders, can help the elderly individual maintain interdental cleansing and health.
Adjunctive to a rigorous oral hygiene regime, three general products can provide relief: rinses, coating agents, and analgesics.2 Rinses serve to cleanse, moisturize, and lubricate. There are a variety of home-mixed preparations, such as salt and/or soda. There is also hydrogen peroxide diluted in water, which is used primarily to debride ulcerated and crusted areas; it is not to be used frequently.
Coating agents are used for sustained moisturizing and lubricating. A very simple one, easily available, is water-soluble lubricating gel; there are also anesthetic agents that can be used alone or with some type of suspension to maintain substantivity or increase time on the mucosa. However, these products should be used with caution and in limited circumstances. Anesthetic gels have a tendency to diffuse throughout the oral cavity and even down the throat; some patients are very uncomfortable with that sensation, and the sensation of having difficulty breathing is common, particularly in an elderly individual who may have challenges swallowing to begin with.
Analgesics for dry mouth include topical analgesics, lidocaine 2% viscous, and in extreme circumstances, systemic analgesics, typically ibuprofen. On rare and extreme occasions, opioid narcotics can be used, but very cautiously because of the gastrointestinal (GI) distress and potential alteration of GI bacteria.1,10
For treating oral infections in patients with xerostomia, there are a variety of medicaments that can be used, again with discretion. Steroid creams and ointments are commonly used with success,52 as well as antibiotics. It is important to culture resistant organisms. Chlorhexidine gluconate is an antimicrobial rinse to be used for brief periods, no more than perhaps 2 weeks at a time, that has some success well.53 However, antibiotics should be prescribed with caution in elderly individuals, with a warning to see their physician as soon as possible on experiencing any GI distress.
When a removable prosthesis is used, it should be treated with a dilute bleach solution to remove any kind of microorganisms that may reside within the acrylic portion. A removable partial denture with metal components should be treated with a commercial tablet or fluid or rinse to clean it.
Preventatives for caries include antimicrobials such as chlorhexidine gluconate, dentifrices, fluoride varnish, and fluorides applied as over-the-counter and prescription rinses or gels. In the author’s opinion, custom trays are highly recommended for gels, and gels are better than foams. Aggressive prevention and frequent recall—as frequent as every 3 months—are necessary, and modifying a patient’s diet to accommodate high caries risk should be discussed. The frequency and amount of refined carbohydrates as well as the duration of exposure of the mouth to refined carbohydrates and acidic substances should be carefully monitored and managed.
There is a large selection of over-the-counter products available to treat xerostomia, including ACT® Dry Mouth mouthwash, lozenges, and toothpaste (Chattem Inc., www.actoralcare.com), Xerostom® gel (Biocosmetics Laboratories, www.biocosmetics.es), Xylitol gum (Epic Dental LLC, www.epicdental.com), and PreviDent® dry mouth rinse (Colgate Professional, www.colgateprofessional.com), and many other mouthwashes, toothpastes, moisturizers, gels, gums, and sprays.53-57
Thirst-stimulation products such as sugarless chewing gum and hard candies can help activate residual viable salivary gland tissue. Some healthcare professionals advocate the use and chewing of hard foods such as carrots or celery, which is also good for healthy snacking. However, patients with dry mouth may have challenges chewing and swallowing these dry hard foods, so recommendation of these tactics is dependent on the context of the whole patient. Pharmacologic stimulation with pilocarpine as well as other sialogogic drugs is well-researched.2,58-61 However, there are secondary effects, including GI alterations, sweating, bronchospasms, altered heart rate, and blurred vision, that can be quite disconcerting.2,58-61
Additional aids include avoiding spicy foods, adding liquid to a diet, making sure that a liquid is available during meals, and humidifying the air—particularly at night when the condition is due to circadian rhythm salivary flow decreases.2 Filtering room air, as well as using lip moisturizers to keep lips moist and comfortable when speaking and functioning, can also be efficacious.2
Conclusion
This overview has endeavored to illuminate the elements that can contribute to salivary gland hypofunction in elderly patients. Detection and recognition are key to reducing the morbidity associated with this condition. After dry mouth is identified, aggressive management is recommended, with a broad armamentarium of commonly available, cost-effective therapeutic aids to leverage in alleviating some of the more severe symptoms of xerostomia and salivary hypofunction.
References
1. Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farré M. Salivary secretory disorders, including drugs, and clinical management. Int J Med Sci. 2015;12(10):811–824.
2. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
3. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69.
4. Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49(2):309-326.
5. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth – 2nd edition. Geriodontology 1997;14(1):33-47.
6. Schneyer LH. Method for the collection of separate submaxillary and sublingual salivas in man. J Dent Res. 1955;34(2):257-261.
7. Villa A, Abati S. Risk factors and symptoms associated with xerostomia: a cross-sectional study. Aus Dent J. 2011;56:290-295.
8. Villa A, Polimeni A, Strohmenger L, et al. Dental patients’ self-reports of xerostomia and associated risk factors. J Am Dent Assoc. 2011;142(7):811-816.
9. Navazesh M, Kumar SK. Xerostomia: prevalence, diagnosis, and management. Compend Contin Educ Dent. 2009;30(6):326-328,331-334.
10. Grisius MM. Salivary gland dysfunction: a review of systemic therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(2):156-162.
11. Menopause. eMedicineHealth. http://www.emedicinehealth.com/menopause/article_em.htm. Accessed June 16, 2017.
12. Dutt P, Chaudhary SR, Kumar P. Oral health and menopause: a comprehensive review on current knowledge and associated dental management. Ann Med Health Sci Res. 2013;3(3):320-323.
13. Steele LS, Glazier RH. Is donepezil effective for treating Alzheimer’s disease? Can Fam Physician. 1999;45:917-919.
14. Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs. 2000;60(5):1095-1122.
15. Finkel SI. Effects of rivastigmine on behavioral and psychological symptoms of dementia in Alzheimer’s disease. Clin Ther. 2004;26(7):980-990.
16. Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330(25):1797-1810.
17. López-Pintor RM, Casañas E, González-Serrano J, et al. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. 2016;2016:4372852.
18. von Bültzinglöwen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl):S57.e1–e15.
19. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554-558.
20. Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63(7):2021-2030.
21. Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca: analysis of 618 patients with suspected Sjögren’s syndrome. Arthritis Rheum. 1994;37(6):869-877.
22. Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012;64(4):475-487.
23. Daniels TE, Criswell LA, Shiboski C, et al. An early view of the International Sjögren’s Syndrome Registry. Arthritis Rheum. 2009;61(5):711-714.
24. Dehydration in adults. eMedicineHealth. http://www.emedicinehealth.com/dehydration_in_adults/article_em.htm. Accessed June 16, 2017.
25. Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47(1):1-12.
26. Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26(9):796-807.
27. Cooper JS, Fu K, Marks J, Silverman S. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31(5):1141-1164.
28. Dirix P, Nuyts S, van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 2006;107(11):2525-2534.
29. Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992;28(2-3):457-462.
30. Scrimger R. Salivary gland sparing in the treatment of head and neck cancer. Expert Rev Anticancer Ther. 2011;11(9):1437-1448.
31. Koga DH, Salvajoli JV, Alves FA. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14(1):40-44.
32. Lin S, Lu JJ, Han L, et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasalpharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10:39.
33. Konstantinidis I, Paschaloudi S, Triaridis S, et al. Bilateral multiple sialolithiasis of the parotid gland in a patient with Sjögren’s syndrome. Acta Otorhinolaryngol Ital. 2007;27(1):41-44.
34. Correia PN, Carpenter GH, Osailan SM, et al. Acute salivary gland hypofunction in the duct ligation model in the absence of inflammation. Oral Dis. 2008;14(6):520-528.
35. Farneti P, Macri G, Gramellini G, et al. Learning curve in diagnostic and interventional sialendoscopy for obstructive salivary diseases. Acta Otorhinolaryngol Ital. 2015;35(5):325-331.
36. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth. Gerodontology. 1986;5(2):75-99.
37. Nederfors T, Nauntofte B, Twetman S. Effects of furosemide and bendroflumethiazide on saliva flow rate and composition. Arch Oral Biol. 2004;49(7):507-513.
38. Saini-Chohan HK, Hatch GM. Biological actions and metabolism of currently used pharmacological agents for the treatment of congestive heart failure. Curr Drug Metab. 2009;10(3):206-219.
39. Mangrella M, Motola G, Russo F, et al. Hospital intensive monitoring of adverse reactions of ACE inhibitors. Minerva Med. 1998;89(4):91-97.
40. Wong KC, Franz DN, Tseng J. Clinical pharmacology of alpha2-agonist and beta-adrenergic blocker. Ma Zui Xue Za Zhi. 1989;27(4):357-362.
41. Zaclikevis MV, D’Agulham AC, Bertassoni LE, et al. Effects of benzodiazepine and pilocarpine on rat parotid glands: histomorphometric and sialometric study. Med Chem. 2009;5(1):74-78.
42. Nathwani NS, Gallagher JE. Methadone: dental risks and preventive action. Dent Update. 2008;35(8):542-544,547-548.
43. Elad S, Heisler S, Shalit M. Saliva secretion in patients with allergic rhinitis. Int Arch Allergy Immunol. 2006;141(3):276-280.
44. Howell G 3rd, West L, Jenkins C, et al. In vivo antimuscarinic actions of the third generation antihistaminergic agent, desloratadine. BMC Pharmacol. 2005;5:13-25.
45. Gonzalo-Garijo MA, Bobadilla P. Ibuprofen-induced fever in Sjogren’s syndrome. J Investig Allergol Clin Immunol. 2006;16(4):266-267.
46. Ciancio SG. Medications’ impact on oral health. J Am Dent Assoc. 2004;135(10):1440-1448.
47. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.
48. Tiwari M. Science behind human saliva. J Nat Sci Biol Med. 2011;2(1):53-58.
49. Valdez IH, Wolff A, Atkinson JC, et al. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer. 1993;71(5):1848-1851.
50. DailyMed. National Institutes of Health. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=16b10412-0031-4e7b-b510-99ae89b138e5. Accessed June 16, 2017.
51. Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49(2):309-326.
52. Fox PC, Datiles M, Atkinson JC, et al. Prednisone and piroxicam for treatment of primary Sjögren’s syndrome. Clin Exp Rheumatol. 1993;11(2):149-156.
53. Simons D, Kidd EA, Beighton D, Jones B. The effect of chlorhexidine/xylitol chewing-gum on cariogenic salivary microflora: a clinical trial in elderly patients. Caries Res. 1997;31(2):91-96.
54. Fox PC. Salivary enhancement therapies. Caries Res. 2004;38(3):241-246.
55. Itthagarun A, Wei SH. Chewing gum and saliva in oral health. J Clin Dent. 1997;8(6):159-162.
56. Makin SA. Stannous fluoride dentifrices. Am J Dent. 2013;26(Spec No A):3A-9A.
57. Nordström A, Birkhed D. Preventive effect of high-fluoride dentifrice (5,000 ppm) in caries-active adolescents: a 2-year clinical trial. Caries Res. 2010;44(3):323-331.
58. Braga MA, Tarzia O, Bergamaschi CC, et al. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int J Dent Hyg. 2009;7(2):126-130.
59. Aframian DJ, Helcer M, Livni D, et al. Pilocarpine treatment in a mixed cohort of xerostomic patients. Oral Dis. 2007;13(1):88-92.
60. Fox PC, van der Ven PF, Baum BJ, Mandel ID. Pilocarpine for the treatment of xerostomia associated with salivary gland dysfunction. Oral Surg Oral Med Oral Pathol. 1986;61(3):243-248.
61. Fife RS, Chase WF, Dore RK, et al. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome. Arch Intern Med. 2002;162(11):1293-1300.
ABOUT THE AUTHOR
Domenica Sweier, DDS
Clinical Associate Professor
University of Michigan School of Dentistry
Ann Arbor, Michigan