You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Peri-implant diseases are initiated by bacteria and are classified as either peri-implant mucositis or peri-implantitis.1,2 Peri-implant mucositis is characterized by soft-tissue inflammation and bleeding on probing, but without peri-implant bone loss on any of the implant surfaces. If left untreated, peri-implant mucositis will progress to peri-implantitis, which is characterized by the same clinical conditions as well as peri-implant suppuration and bone loss surrounding one or more implant surfaces.3
The goal of peri-implant disease treatment, therefore, is to stop the inflammatory process and halt and ideally reverse the bone loss resulting from the disease process. Peri-implant mucositis can be successfully treated nonsurgically to reverse the infection.4 Nonsurgical treatments have included establishing excellent oral hygiene and removing biofilm deposits. Among the methods for achieving biofilm removal around dental implants is air polishing.
Air Polishing
Evolving from technology originally developed in 1945 by Dr. Robert Black,5,6 standard air-polishing devices, also known as air-powder polishing devices, propel a pressurized air/water fluid stream containing abrasive particles by kinetic energy through a handpiece nozzle against the tooth surface, thereby removing dental plaque and stain.7 Air polishing is intended for dental plaque and stain removal only, not the removal of hard deposits; air abrasion is used for the removal of decayed enamel and roughening dental material surfaces before bonding.7-9
An abrasive powder is required with air-polishing devices, which typically include a delivery system with two concentric tubes. The central tube carries the air laden with the abrasive particles, while the outer tube transports the water stream (Figure 1). The water stream acts as a shroud to limit the dispersion of the air stream, and the two are directed toward the tooth surface on which the polishing slurry of air/powder and water is formed. Currently, a specially processed sodium bicarbonate powder is the most commonly used for subgingival applications, but other powders are available for other indications (Table 1).10
The powder is combined with a flavoring agent and small amounts of calcium phosphate and silica to facilitate a free flow within the device. The average sodium bicarbonate particle size is 74 µm, and its hardness is 2.5 on the Mohs scale, which ranges from 1 to 10. Comparatively, pumice, the standard particle used in prophylaxis paste, has a Mohs hardness number of 6,11 and air abrasion with aluminum oxide is about four times more abrasive than air polishing with sodium bicarbonate (Table 2).8,9
Applications for Peri-implant Diseases
Air polishing has been studied in the treatment of peri-implant diseases, in both a nonsurgical approach in the treatment of peri-implant mucositis and an open surgical approach for the treatment of peri-implantitis. Standard air polishing of submucosal implant surfaces by a nonsurgical approach for the treatment of peri-implantitis does not appear to reduce the inflammatory condition or bacterial load in the long term and has limited advantage over other mechanical debridement methods.12-14 In one study examining patients with early to moderate peri-implantitis, nonsurgical treatment with either glycine air polishing or carbon curettes and subgingival delivery of chlorhexidine resulted in improvement in both groups, but the improvements were not sustained long term. Interestingly, however, more improvement in bleeding index was observed in the glycine air-polishing–treated group.15
In fact, complete biofilm elimination is difficult to achieve using nonsurgical means, and reosseointegration may be difficult, if not impossible, to attain without surgical access to ensure thorough debridement of the peri-implantitis defect and detoxification of the implant surface.16 However, when combined with a surgical approach, the application of air polishing as an implant-cleaning method can result in significant improvement in the clinical parameters of marginal bleeding, bleeding on probing, suppuration, and probing depth, when reinforced with regular good hygiene.17 Additionally, air polishing with sodium bicarbonate in conjunction with resin curettes was shown to result in significant improvement in all clinical parameters.18
Chemical agents (eg, citric acid, stannous fluoride, tetracycline HCl, chlorhexidine gluconate, hydrogen peroxide, chloramine T, or sterile water) have also been used for dental implant detoxification in the treatment of peri-implant diseases.12 Compared with these methods, in an in vitro study, air polishing was reported to be more effective in removing bacterial endotoxin.19
Unfortunately, standard air-polishing units have been shown by several studies to cause alterations to titanium implant surfaces, which can result in increased plaque adherence. When air polishers using sodium bicarbonate were investigated for cleaning implant surfaces, surface defects and alterations were recorded. As a result, standard sodium bicarbonate air-polishing devices are often not recommended for cleaning around implants.20
An Alternative Approach for Air Polishing Implants
The delivery arrangement of standard air-polishing devices with two concentric tubes can lead to frequent blockages, because a hard, crystalline deposit of abrasive anhydrous sodium bicarbonate can accumulate in the lumen of the air tube.21 The device then cannot function, resulting in clinical downtime while the nozzle is either replaced or cleaned. Additionally, and perhaps most importantly, standard air-polishing devices bombard surfaces (eg, tooth or implant) with potentially damaging abrasive particles, because no mixing with water occurs until the particles and water hit the surface.
In contrast, air polishers with homogenous stream technology (HST) premix the air and particle stream with the water stream to form the polishing slurry within the nozzle before emission from a single orifice, not on the tooth or implant surface (Figure 2). Such air-polishing technology eliminates the downtime frequently associated with traditional air-polishing devices, because constant flushing of the single exit orifice prevents buildup of crystalline deposits and, therefore, blockage. Additionally, because the sodium bicarbonate particles are soluble, surface softening of the individual particles results when the air stream is mixed with the water stream within the nozzle; the resultant slurry is gentler but produces a more efficient cleaning action than the dry particles associated with standard air-polishing devices. Further, the efficiency of the system enables its operation with less air pressure.22
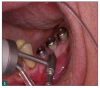
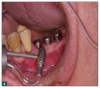
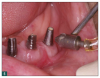
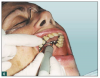
As a result, innovative air-polishing devices utilizing HST or glycine powder can be used routinely for implant maintenance, biofilm removal around implants (Figure 3 and Figure 4), and cleaning around implant abutments (Figure 5), attachments, and superstructures (Figure 6 and Figure 7) without concern about damaging the implant (eg, titanium implants) or transmucosal element surfaces. In fact, an HST polishing system and a glycine powder air-polishing system were shown to cause no damage to titanium implant surfaces during cleaning.23
When combined with surgical access, HST or glycine air-polishing devices have been shown to produce clean implant surfaces, as viewed by scanning electron microscope (SEM)24; a bacteria-free surface; and increased cell attachment to test substrate samples.25 However, sodium bicarbonate powder resulted in higher cell attachment compared with glycine powder.
Although every air-polishing agent has the potential to cause some gingival erosion, trauma to soft tissues from air polishing has been shown to heal rapidly after treatment and to be of little clinical significance. In healthy patients with healthy gingiva or slight gingivitis, there was no significant clinical difference between the prophy cup and paste method and the air-polishing method.6,23,26,27
Caveats for Air Polishing
To prevent facial emphysema, which has been associated with oral lacerations as small as 4 mm and results from compressed air becoming trapped in interstitial spaces, air-polishing devices should not be directly aimed subgingivally. The most common causes of facial emphysema after dental procedures are consequences of high-speed handpiece use during third-molar extractions28 or the use of air/water syringes near extraction or surgical sites or lacerations.29,30 Facial emphysemas have also been reported after air polishing.31
Therefore, incorrect angulation of the air-polisher handpiece must be avoided to prevent facial emphysema.6,11,15 Air-polishing handpiece nozzles should never be directed into or near traumatic lacerations or surgical wounds where the intraoral barrier is disrupted or into extraction sites. The nozzle should always be directed away from flap margins, which should be protected.
Additionally, the air-polishing technique is contraindicated for use with patients suffering from conditions that can result in breathing difficulties (eg, chronic pulmonary diseases or chronic asthma), which may be exacerbated by inhaling the polishing spray. Patients with difficulty swallowing, those with a communicable disease that might be transmitted by the aerosol to attending staff, or patients whose immune system is compromised also should not be treated with air polishing.
Other contraindications include patients taking potassium supplements, antidiuretics, or steroid therapy, as well as patients with terminal-stage renal disease or Addison or Cushing disease. Patients on a restricted sodium diet for hypertension have also been included in the contraindications for air polishing, although it remains unclear whether the amount of sodium ingested during air-polishing procedures is sufficient to cause elevated blood pressure, alkalosis in the bloodstream, or an increase in sodium blood level; nevertheless, sodium-free air-polishing abrasive substitutes include glycine, calcium carbonate, and aluminum trihydroxide.6,7
Conclusion
Peri-implant diseases can be successfully treated using HST air-polishing devices, both nonsurgically (ie, for peri-mucositis) and in combination with surgical flaps (ie, for peri-implantitis) to remove biofilm deposits, stop the inflammatory process, and, ideally, reverse bone loss from the infection. Air polishing—whether using HST devices and sodium bicarbonate, or glycine powder devices—is less time-consuming for subgingival biofilm removal and can contribute to significant improvement in the clinical parameters of marginal bleeding, bleeding on probing, suppuration, and probing depth. To prevent the risk of facial emphysema, care must be taken not to directly aim the abrasive stream subgingivally.
REFERENCES
1. Gilman RS, Maxey BR. The effect of root detoxification on human gingival fibroblasts. J Periodontol. 1986;57(7):436-440.
2. Worrall SF, Knibbs PJ, Glenwright HD. Methods of reducing bacterial contamination of the atmosphere arising from use of an air-polisher. Br Dent J. 1987;163(4):118-119.
3. Rayman S, Dincer E. Air polishing. Hygiene. 2012;1:7-12.
4. Valderrama P, Blansett JA, Gonzalez MG, et al. Detoxification of implant surfaces affected by peri-implant disease: an overview of non-surgical methods. Open Dent J. 2014;8:77-84.
5. Black RB. Technique for non-mechanical preparation of cavities and prophylaxis. J Am Dent Assoc. 1945;32(15):955-965.
6. Gutmann ME. Air polishing: a comprehensive review of the literature. J Dent Hyg. 1998;72(3):47-56.
7. Barnes CM. Air polishing: a mainstay for dental hygiene. Pennwell Publications. 2013. https://www.dentalacademyofce.com/courses/2423/PDF/1305cei_Barnes_RDH_final.pdf. Accessed May 3, 2017.
8. American Dental Hygienists’ Association. Position paper on the oral prophylaxis. 1998. https://www.adha.org/resources-docs/7115_Prophylaxis_Postion_Paper.pdf. Accessed May 3, 2017.
9. Glossary of Periodontal Terms. 3rd ed. Chicago, IL: American Academy of Periodontology; 1992:40.
10. Christensen R. Oral prophylaxis: prophy-jet. Clin Res Assoc Newsletter. 1981;5:1-5.
11. Petersilka GJ. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol 2000. 2011;55(1):124-142.
12. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-259.
13. Duarte PM, de Mendonça AC, Máximo MB, et al. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol. 2009;80(2):234-243.
14. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
15. Charalampakis G, Leonhardt Å, Rabe P, et al. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicenter study. Clin Oral Implants Res. 2012;23(9):1045-1054.
16. Pippin DJ, Crooks WE, Barker BF, et al. Effect of an air-powder abrasive device used during periodontal flap surgery in dogs. J Periodontol. 1988;59(9):584-588.
17. Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282-285.
18. Zablotsky MH, Diedrich DL, Meffert RM. Detoxification of endotoxin-contaminated titanium and hydroxyapatite-coated surfaces utilizing various chemotherapeutic and mechanical modalities. Implant Dent. 1992;1(2):154-158.
19. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
20. Mouhyi J, Sennerby L, Pireaux JJ, et al. An XPS and SEM evaluation of six chemical and physical techniques for cleaning of contaminated titanium implants. Clin Oral Implants Res. 1998;9(3):185-194.
21. Cooper MD, Wiechmann L. Essentials of Dental Hygiene: Clinical Skills. Upper Saddle River, NJ: Pearson; 2006.
22. Johnson-Promident. H.S.T. technology. http://johnson- promident.com/h-s-t-technology/. Accessed May 12, 2017.
23. Barnes CM, Fleming LS, Mueninghoff LA. SEM evaluation of the in-vitro effects of an air-abrasive system on various implant surfaces. Int J Oral Maxillofac Implants. 1991;6(4):463-469.
24. Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011;38(9):872-878.
25. Persson GR, Roos-Jansåker AM, Lindahl C, Renvert S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2011;82(9):1267-1278.
26. Petersilka G, Faggion CM Jr, Stratmann U, et al. Effect of glycine powder air-polishing on the gingiva. J Clin Periodontol. 2008;35(4):324-332.
27. Kozlovsky A, Artzi Z, Nemkovsky CE, Hirshberg A. Effect of air-polishing devices on the gingiva: histological study in the canine. J Clin Periodontol. 2005;32(4):329-334.
28. Sekine J, Irie A, Dotsu H, Inokuchi T. Bilateral pneumothorax with extensive subcutaneous emphysema manifested during third molar surgery. A case report. Int J Oral Maxillofac Surg. 2000;29(5):355-357.
29. Tan WK. Sudden facial swelling: subcutaneous facial emphysema secondary to use of air/water syringe during dental extraction. Singapore Dent J. 2000;23(1 suppl):42-44.
30. Uehara M, Okumura T, Asahina I. Subcutaneous cervical emphysema induced by a dental syringe: a case report. Int Dent J. 2007;57(4):286-288.
31. Finlayson RS, Stevens FD. Subcutaneous facial emphysema secondary to use of the Cavi-Jet. J Periodontol. 1988;59(5):315-317.