You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Peri-implant diseases, which are defined as inflammatory processes in the tissues surrounding an implant, include peri-implant mucositis (ie, reversible, involving functional implants) and peri-implantitis (ie, not limited to soft tissue, but also characterized by loss of peri-implant bone).1 Although variable, studies have shown that peri-implant diseases are prevalent among patients who have received dental implant treatments. Peri-implant mucositis has occurred in 80% of patients and 50% of implant sites in two cross-sectional studies, while peri-implantitis was identified in 28% and 56% of the patients studied.2 In particular, peri-implantitis has been prevalent in 10% of implant sites and 20% of patients during the 5 to 10 years following implantation.2,3
These numbers imply a worrisome trend. An independent survey predicts that in the US dental implant market alone, more than 2.5 million dental implants will have been placed in 2016.4 If 10% of these develop peri-implantitis, dentists could be presented with 257,000 implants with peri-implantitis. Over a 5-year time period, that translates to 1.2 million diseased dental implants.
Therefore, clinicians should understand the etiology and risk factors for peri-implant diseases (eg, peri-implant mucositis, peri-implantitis), and how to properly diagnose and treat each. Further, to avoid any potential negative outcomes from dental implant treatments, critically evaluating and managing patients will be essential to prevent the development of peri-implant diseases following implant placement.
Etiology of Peri-Implant Diseases
Peri-implant mucositis (ie, gingival inflammation surrounding the teeth or peri-implant sites) results from bacterial plaque buildup.5 Although reversible at the biomarker level, gingivitis and peri-implant mucositis require more than 3 weeks of resumed plaque control and healing to achieve and establish peri-implant mucosal health.6
Fortunately, just as not all sites with gingivitis will develop periodontitis, not all sites with peri-implant mucositis will develop peri-implantitis.7,8 However, when the lesions in the plaque-associated peri-implant tissue involve connective tissue and alveolar bone and are allowed to progress, loss of the implant may occur.9,10
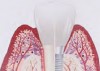
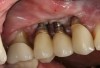
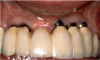
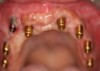
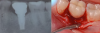
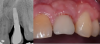
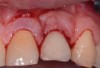
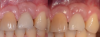
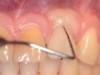
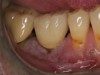
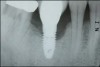
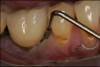
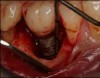
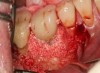
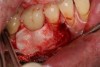
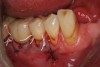
The entry of inflammatory cell infiltrate into the connective tissue (Figure 1 and Figure 2) differentiates peri-implantitis from peri-implant mucositis.1 Unlike a natural tooth—where periodontal fibers, transepithelial fibers, and gingival fibers absorb this exudate—none of these fibers are present around an implant (Figure 3). This allows the inflammatory cell exudate to directly enter the bone, resulting in bone loss and concomitant soft-tissue loss (Figure 4).1
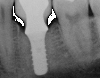
Peri-implantitis-associated bone loss varies. In some individuals, bone breakdown incorporates rapidly, while in others it occurs gradually.11 Bone loss is characterized by a nonlinear progression (eg, not 0.5 mm bone loss one year, then 0.5 mm bone loss the following year, etc), with the rate of loss increasing over time. This necessitates early detection and treatment, since peri-implantitis advances rapidly (Figure 5 and Figure 6).11
Risk Factors for Peri-Implant Diseases
Seven risk factors are associated with peri-implant diseases: oral hygiene, history of periodontitis, cigarette smoking, diabetes with poor metabolic control, alcohol consumption, genetic traits, and implant surface.2 The ability to clean implants is significant to plaque control, and studies have shown that peri-implantitis developed in 48% of implants where there was no accessibility for cleaning, versus 4% when plaque control was possible.12When mucositis is present within 5 years, 44% of those implants develop peri-implantitis; if it is absent, only 18% develop peri-implantitis.13 Patients with a history of periodontitis and cigarette smoking have shown an association with mucositis, putting them at further risk for developing peri-implantitis.14 Additionally, although data is limited, research demonstrates that regardless of whether the surface is machined or roughened, no implant is immune to peri-implantitis.15
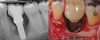
This list of risk factors for peri-implant diseases has recently been expanded to include poor prosthetic fit and micromotion (Figure 7), poor implant positioning (Figure 8 and Figure 9), occlusion, retained cement (Figure 10), possibly hypertension, inadequate gingiva growth and development, reuse of healing abutments, and foreign particles (eg, titanium).16-26 Poorly fitting restorations contribute to peri-implant diseases by interfering with daily hygiene efforts; accumulating plaque on open margins; and exposing cement that encourages pathogenic bacteria, thereby causing micromotion of the restoration, leading to increased inflammation and crestal bone loss.16,19-21
Light marginal ridge contacts allow food impaction and occlusal trauma to develop; when these circumstances occur, the different dynamics between teeth and implants have been ignored.16,19 When implants are improperly positioned (eg, too far buccally), results could include buccal bone resorption, subsequent recession, and exposure of rough implant surfaces, which encourages biofilm development and peri-implantitis.16,17 Additionally, disregarding the potential need for ongoing occlusal adjustments to ensure favorable functional dynamics can further contribute to peri-implant diseases (ie, by creating circumstances conducive to implant loosening, crestal bone loss, and biofilm development).16,19 Unlike natural teeth, implants do not move, thereby necessitating occlusal adjustments—as warranted—at each patient recall appointment.
Retained cement is a growing area of concern that is increasingly cited in the literature as a risk factor for peri-implant diseases.18,20,21 Endoscopic examination of implants with peri-implant disease revealed that 81% of cases were associated with cement that required removal via a flap approach.20 Only 74% of those implants achieved disease resolution, and 26% still had problems.20
Residual cement also increases the likelihood of developing peri-implantitis in patients with a history of periodontitis.21 In fact, research has documented that in cases with a history of periodontitis where there was residual cement, 100% developed peri-implant disease compared to patients without a history of periodontitis, fewer of which developed peri-implant disease.21
However, the presence of adequate gingiva surrounding endosseous dental implants is essential for resistance to inflammatory insults that may be present.24,25 Reduced keratinized mucosa (ie, gingiva) around implants appears to be associated with the clinical parameters accompanying inflammation and poor oral hygiene.24 A lack of adequate gingiva around endosseous dental implants is associated with more plaque accumulation, tissue inflammation, mucosa recession, and attachment loss.25
For this reason, growth and development of the gingival tissues—which occur throughout an individual’s lifetime—are significant considerations and risk factors for peri-implant diseases.22,23 For example, vertical and horizontal changes can occur into age 60+,27 and such growth and development could cause the bone or teeth to shift downward, resulting in implants that appear labially angled and ankylosed. When bone recedes on the facial, rough implant threads could be exposed, which predisposes patients to inflammation and developing peri-implantitis.16
Preventing Peri-Implant Diseases
Conscientious oral hygiene, proper implant spacing/placement, removal of residual cement, and professional maintenance are measures for preventing peri-implant diseases.28 In addition to screening implant candidates for other risk factors, ensuring patients have active plaque control and are capable of maintaining proper oral hygiene is essential.28 Adequate plaque control should be established at home prior to implant placement. Additionally, presurgical antibiotics—although still debated—have been shown to increase implant survival rates.28,29
To ensure proper implant spacing/placement, considering the implant’s ultimate 3-dimensional position and using a surgical template are requisites for predictability.17 Orofacially, at least 2 mm of buccal plate of bone is desired, and the implant should merge toward the cingulum. Mesial-distally, at least 3 mm between implants and 2 mm between an implant and an adjacent tooth is recommended.17 In an apico-buccal perspective, an implant that is 2 mm to 3 mm apical to the cemento-enamel junction (CEJ) of the adjacent tooth—if there is no gingival recession—is advised.17 In the presence of recession, the implant should be placed 3 mm apical to the anticipated gingival margin.17
These exacting positions can be ascertained by working from the top down. Collaborating with the restorative dentist to fabricate a stent and an accurate guide of where the teeth/restorations should end will enable placement of the implant 3 mm apical to the CEJ.17
Such an approach has implications for facilitating removal of—if not altogether preventing—residual cement. The deeper the implant-supported restorations are, the more likely it is that residual cement will remain.19,20 Compounding the problem is the fact that not all cements can be seen radiographically (eg, deep and on the buccal aspect).27 Therefore, cementing crowns extraorally to extrude the cement is advised.19,20,27
Diagnosing Peri-Implant Diseases
Early and accurate diagnosis of peri-implant diseases is predicated on specific reliable diagnostic indicators (eg, bleeding on probing, suppuration).30,31 The absence of bleeding on probing around implants indicates healthy peri-implant tissue, although debate continues regarding whether probing dental implants is prudent.30,32 However, when probing implants, a rounded, 25 Ncm probe is recommended. The soft-tissue seal inhibits probing depth penetration both in healthy and slightly inflamed peri-implant tissue, but not in peri-implantitis. Therefore, if bleeding on probing occurs and there are significant probing depths (eg, 5 mm to 8 mm), the site should be treated as early as possible.31,32
Because an implant can lose between 70% and 90% of bone—yet still not be mobile—mobility is not a good diagnostic indicator of peri-implantitis.33 Additionally, because radiographs may produce too many false negatives, and based on their low sensitivity for detecting early changes, they are not reliable diagnostic indicators of peri-implantitis.33 Therefore, viewing radiographs over time to observe the bone level and any changes is advisable to determine when and if disease is present and treatment should be initiated.31,33
Treatments for Peri-Implant Diseases
Successfully treating peri-implant diseases (ie, peri-implant mucositis, peri-implantitis) is dependent upon identification and classification of the disease and its severity (ie, early, moderate, advanced), respectively. For example, an 8-mm long implant with 4 mm of bone loss may be a more severe case than a 13-mm implant with the same amount of bone loss. Other aspects indicating disease severity include bleeding on probing, suppuration, and pocket depths.34-36
Peri-implant mucositis has typically been treated by removing biofilm from occlusions, removing residual cement, and administering adjunctive plaque control therapies.37,38 This may require local anesthesia, sometimes flaps, remaking the prosthesis with adequate embrasures, and/or re-preparing the abutments to provide sufficient space for better at-home oral hygiene. However, studies have indicated that professionally administered plaque control efforts and adjunctive agents (eg, antimicrobials, systemic antibiotics such as azithromycin) do not improve the efficacy of peri-implant mucositis treatment, and a low percentage of cases treated with adjunctive therapies have realized effective disease resolution.38,39
Fortunately, newer techniques (eg, laser assisted peri-implant mucositis [LAPIM] treatments) may enhance the success of peri-implant disease treatments. Using a neodymium-YAG laser and the LAPIM procedure has yielded better results (Figure 11 through Figure 14).40-42
Treatment for peri-implantitis, on the other hand, has been described systematically to include approaches ranging from mechanical debridement to explantation based upon pocket depth.2,29 In particular, mechanical debridement is recommended when pockets are 3 mm to 4 mm; additional antiseptic treatment when pockets are 4 mm to 6 mm; antibiotic therapy when pockets are >6 mm; resective regenerative therapy when prior treatments are ineffective; and explantation when pockets are >8 mm, with bleeding and pain).2,29
Removal of the implant—along with rebuilding the site and placing a new implant—is commonly suggested when bone loss equal to two-thirds of the implant length has occurred.32 Unfortunately, there is little validation in the literature to substantiate this recommendation.43 In fact, the available evidence does not support any specific recommendations for peri-implantitis therapy.43 Even the application of grafting materials and barrier membranes, which has resulted in greater pocket depth reduction and radiographic bone fill, lacks high-quality comparison studies to support its efficacy.44
Despite the lack of clear-cut, evidence-based recommendations, the most common treatments for peri-implantitis have been found to be periodontal flap surgery with oestoplasty, which has been performed 47% of the time; regenerative therapy 20% of the time. Regardless of the treatment, the overall success rate at the patient level has been 69% (ie, 31% failure rate).45 Interestingly, the effectiveness of the selected peri-implantitis therapy was found to be impaired by severe periodontitis, indicating that treating the periodontitis prior to placing the implant is essential.45
Access surgery combined with surface decontamination (eg, chemical agents, air abrasives, lasers) and antibiotics are also being used to treat peri-implantitis, but with only about a 60% rate of successful resolution at the treated site(s) (ie, 40% failure rate).2,29 However, consistent with other findings, no single method for surface decontamination has been found to be superior.2,46 In fact, at this time, all techniques and agents have been shown to be equally effective or ineffective for detoxifying contaminated implant surfaces.46
A Combination Approach
Recently, the literature has cited an approach for successfully managing peri-implantitis that combines mechanical and chemical methods for decontamination that encompasses eight specific steps.36,40,41 These steps include: critically evaluated case selection (Figure 15 through Figure 17); one-flap access (Figure 18); surface decontamination; defect debridement; placement of a biologic on the cleaned implant surface; filling defect with mineralized freeze-dried bone and/or using anorganic bovine bone (Figure 19); coverage with an absorbable membrane or subepithelial connective tissue graft (Figure 20); and coronal positioning of the flap (Figure 21). A subsequent requisite step is professional maintenance (Figure 22).40,41
The successful outcomes achieved with this approach are credited to diligent case selection.36 For example, radiographic examination may reveal that the defect on the implant extends to the apex, or that surface decontamination would need to go around the apex of the implant. Surface decontamination alone in this case could result in paresthesia. Therefore, this represents a scenario in which implant removal may be prudent.40,41
Also contributing to the success of this approach is the manner in which surface detoxification is completed.36 An air abrasive with powder glycine is used for 1 to 2 minutes, followed by a sterile saline alone for 1 to 2 minutes to wash away the powder. Then, minocycline, slurry, or citric acid is placed all around the implant surface. Although there has been concern regarding air embolisms resulting from the use of air abrasives, blocking off the flap with non-woven gauze during this procedure prevents the powder from reaching underneath the flap.40,41
In 3- to 7.5-year follow-ups, radiographs have demonstrated that this combined approach to per-implantitis treatment has resulted in more than 3.7 mm of radiographic bone fill sounding, over 3 mm of new bone from sounding, pocket depth reduction average of more than 5 mm, and more than 1 mm coronal apposition of the soft tissue.36
Conclusion
Despite the increasing prevalence of peri-implant diseases, the literature indicates that when problems such as bleeding on probing and bone loss are identified and treated in their early stages, the progression of disease can be arrested or ameliorated. Therefore, routine implant maintenance and monitoring are essential for preventing peri-implant mucositis and/or its progression to peri-implantitis. However, even before the implants are placed, it behooves dentists to understand the risk factors for peri-implant diseases to avoid patient- and implant placement-related characteristics that could contribute to negative outcomes.
ABOUT THE AUTHORS
Stuart J. Froum, DDS
Clinical Professor and Director of Clinical Research, Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York; Private Practice, New York, New York
Paul S. Rosen, DMD, MS
Clinical Associate Professor of Periodontics, University of Maryland Dental School, Baltimore, Maryland; Private Practice, Yardley, Pennsylvania
REFERENCES
1. Albrektsson TO, Johansson CB, Sennerby L. Biological aspects of implant dentistry: osseointegration. Periodontol 2000. 1994;4:58-73.
2. Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282-285.
3. Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23 suppl 6:67-76.
4. iData Research, Inc. 2013.
5. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-259.
6. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
7. Marinello CP, Berglundh T, Ericsson I, et al. Resolution of ligature-induced peri-implantitis lesions in the dog. J Clin Periodontol. 1995;22(6):475-479.
8. Ericsson I, Persson LG, Berglundh T, et al. The effect of antimicrobial therapy on periimplantitis lesions. An experimental study in the dog. Clin Oral Implants Res. 1996;7(4):320-328.
9. Lindhe J, Berglundh T, Ericsson I, et al. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3(1):9-16.
10. Gotfredsen K, Berglundh T, Lindhe J. Bone reactions at implants subjected to experimental peri-implantitis and static load. A study in the dog. J Clin Periodontol. 2002;29(2):144-151.
11. Fransson C, Tomasi C, Pikner SS, et al. Severity and pattern of peri-implantitis-associated bone loss. J Clin Periodontol. 2010;37(5):442-448.
12. Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20(2):169-174.
13. Costa FO, Takenaka-Martinez S, Cota LO, et al. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39(2):173-181.
14. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33(4):296-301.
15. Renvert S, Polyzois I, Claffey N. How do implant surface characteristics influence peri-implant disease? J Clin Periodontol. 2011;38 suppl 11:214-222.
16. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
17. Salama H, Salama MA, Li TF, et al. Treatment planning 2000: an esthetically oriented revision of the original implant protocol. J Esthet Dent. 1997;9(2):55-67.
18. Linkevicius T, Vindasiute E, Puisys A, et al. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin Oral Implants Res. 2013;24(1):71-76.
19. Merin RL. Repair of peri-implant bone loss after occlusal adjustment: a case report. J Am Dent Assoc. 2014;145(10):1058-1062.
20. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
21. Linkevicius T, Puisys A, Vindasiute E, et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24(11):1179-1184.
22. Albert AM, Ricanek K Jr., Patterson E. A review of the literature on the aging adult skull and face: Implications for forensic science research and applications. Forensic Science International. 2007;172(1):1-9.
23. Daftary F, Mahallati R, Bahat O, Sullivan RM. Lifelong craniofacial growth and the implications for osseointegrated implants. Int J Oral Maxillofac Implants. 2013;28(1):163-169.
24. Gobbato L, Avila-Ortiz G, Sohrabi K, et al. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implants. 2013;28(6):1536-1545.
25. Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol. 2013;84(12):1755-1767.
26. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15.
27. Wadhwani C, Rapoport D, La Rosa S, et al. Radiographic detection and characteristic patterns of residual excess cement associated with cement-retained implant restorations: a clinical report. J Prosthet Dent. 2012;107(3):151-157.
28. Wilson TG Jr, Valderrama P, Rodrigues DB. The case for routine maintenance of dental implants. J Periodontol. 2014;85(5):657-660.
29. Lang NP, Lindhe J. Clinical Periodontology and Implant Dentistry. Vol 2. 5th ed. Oxford, UK: Wiley Blackwell: 2008. Chapter 60.
30. Mombelli A, Lang NP. Clinical parameters for the evaluation of dental implants. Periodontol 2000. 1994;4:81-86.
31. Decker AM, Sheridan R, Lin GH, et al. A Prognosis System for Periimplant Diseases. Implant Dent. 2015;24(4):416-421.
32. Parma-Benfenati S, Roncati M, Tinti C. Treatment of peri-implantitis: surgical therapeutic approaches based on peri-implantitis defects. Int J Periodontics Restorative Dent. 2013;33(5):627-633.
33. Brägger U, Hugel-Pisoni C, Bürgin W, et al. Correlations between radiographic, clinical and mobility parameters after loading of oral implants with fixed partial dentures. A 2-year longitudinal study. Clin Oral Implants Res. 1996;7(3):230-239.
34. Okayasu K, Wang HL. Decision tree for the management of periimplant diseases. Implant Dent. 2011;20(4):256-261.
35. Padial-Molina M, Suarez F, Rios HF, et al. Guidelines for the diagnosis and treatment of peri-implant diseases. Int J Periodontics Restorative Dent. 2014;34(6):e102-e111.
36. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
37. Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol. 2015;42 suppl 16:S202-S213.
38. Heitz-Mayfield LJ, Needleman I, Salvi GE, Pjetursson BE. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int J Oral Maxillofac Implants. 2014;29 suppl:346-350.
39. Hallström H, Persson GR, Lindgren S, et al. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J Clin Periodontol. 2012;39(6):574-581.
40. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: A consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863.
41. Froum SJ, Rosen PS. Reentry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 2014;34(1):47-59.
42. Nevins M, Nevins ML, Yamamoto A, et al. Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int J Periodontics Restorative Dent. 2014;34(4):461-466.
43. Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants.2014;29 suppl:325-345.
44. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041.
45. Lagervall M, Jansson LE. Treatment outcome in patients with peri-implantitis in a periodontal clinic: a retrospective study. J Periodontol. 2013;84(10):1365-1373.
46. Suarez F, Monje A, Galindo-Moreno P, Wang HL. Implant surface detoxification: a comprehensive review. Implant Dent. 2013;22(5):465-473.