You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The prevalence of gastroesophageal reflux disease (GERD) in the United States is estimated to be 18.1% to 27.8% accounting for over 8.9 million primary care visits annually.1,2 GERD is a chronic or longer lasting form of gastroesophageal reflux.3 GERD occurs when the lower esophageal sphincter (LES), a group of muscles at the lower end of the esophagus, relaxes and allows the stomach’s contents to flow up into the esophagus or beyond, into the oral cavity (including larynx) or lung.3,4 Gastric acid has a pH of 1.2, which can damage the tissue lining of the esophagus with repeated exposures.1 A diagnosis of GERD is made using a combination of indicators, including self-report of heartburn and regurgitation, endoscopy, or monitoring of reflux in an outpatient setting.3
GERD is common in a number of conditions including post-bariatric surgery, obesity, irritable bowel syndrome, developmental disorders, asthma, sleep apnea, obesity, and pregnancy.5,6 Heartburn and regurgitation are typical symptoms of GERD, although some adults with GERD are asymptomatic.4 GERD symptoms may differ from person to person and range from mild to severe and can include a chronic dry cough, wheezing, asthma, recurrent pneumonia, sinusitis, nausea, vomiting, sore throat, chronic hoarseness or laryngitis, difficulty or painful swallowing, pain in the chest or the upper abdomen, dental erosion, and oral malodor.3,4
The symptoms are influenced by daily activities including diet, stressors and drugs, which can make the assessment of GERD symptoms at one point in time challenging.7 For individuals with disruptive GERD (daily symptoms) sleep may be disturbed and quality of life may be impacted resulting in missed work and/or reduced work productivity.3
Oral symptoms and complications associated with GERD may include nonspecific burning sensation, mucosa ulceration and erosion, erythema of the soft/hard palate mucosa and uvula, loss of taste and either xerostomia or increased salivary flow.1,8 Untreated or unmanaged GERD is capable of long-term complications such as dysphagia, difficulty breathing and esophagitis.4 Esophagitis is an irritation of the esophagus that can lead to precancerous changes or dysplasia and Barrett’s esophagus.9 Barrett’s esophagus is a condition where the tissue of the esophagus is replaced by tissue found in the lining of the intestine and can lead to a rare and deadly cancer, EAC.9 Adenocarcinoma is the leading cause of esophageal cancers in the United States, constituting 80% of the cases, and rendering it the fastest growing cancer in the United States, according to the National Cancer Institute.10,11 The International Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON) found the risk of EAC is five times higher with increased frequency and duration of exposure to symptoms of heartburn and/or regurgitation.12
Lifestyle modifications are an essential part of managing GERD. Dietary factors associated with reflux symptoms include white bread, chocolate, mint, cinnamon, carbonated beverages, fatty foods, alcohol, wine and beer.3 Smoking is also a risk factor for GERD.3 The health professional should provide support for weight loss to attain a healthy weight, cessation of tobacco and alcohol, elevation of the head while sleeping, avoidance of foods that cause symptoms, and avoid eating before bed.3 If lifestyle modifications fail to manage the GERD, medications like histamine-receptor antagonists such as Pepcid® or Zantac® or proton pump inhibitors like Prilosec® may be recommended.3 It is important that use of medications be monitored by medical providers to ensure management of the reflux to prevent long-term complications.
Considering 79% to 87% of patients have persistent symptoms, the use of a GERD screening tool in the oral healthcare setting would provide a simple approach to identify the presence and severity of symptoms.7 Symptoms of GERD can be burdensome on quality of life and can lead to severe, life-threatening complications.3 Moreover, screening would increase the awareness of the burden and risk of cancer imposed by GERD. Oral healthcare professionals’ utilization of a GERD screening tool can be effective in recognizing the early symptoms of GERD. Screenings would encourage interprofessional collaboration between medical and dental healthcare providers to work together to increase awareness of GERD symptoms and manage the associated oral and systemic complications and ultimately improve overall health outcomes. The purpose of this study was to assess the feasibility of using a GERD screening in the dental clinical setting to identify and refer patients.
Methods
This study received approval from the Massachusetts College of Pharmacy and Health Sciences (MCPHS) University’s Institutional Review Board. Patients, 18 years and older, were solicited between January 2014 through March 2014 from two dental hygiene clinics, the first location in Boston, Massachusetts, and the second in Worcester, Massachusetts.
Dental hygiene students and faculty were calibrated and upon patient consent, the questionnaire was administered. Students administered a previously validated, GERD diagnostic screening questionnaire with known enumerated sensitivity (61.4%, 95% CI: 49% to 72.8%) and specificity (96.2%, 95% CI: 91.4% to 98.8%) parameters for GERD diagnosis. Permission to use the GERD screening questionnaire was obtained by authors of said instrument.13 Data obtained in the questionnaire included demographics (age, gender, pregnancy status, history of gastrointestinal disease, and surgeries in the past two years), and six questions relating to GERD symptoms used to construct the diagnostic test for GERD diagnosis as shown in Table 1. Responses to the six questions in Table 1 were used to construct the diagnostic test for GERD diagnosis, Positive (+) GERD test was calculated for each patient as per Offman et al with (a) the presence of heartburn or regurgitation ≥ “about once per week” with associated severity level ≥ “moderate”; or (b) the presence of heartburn or regurgitation ≥ “several times per week” with associated severity level ≥ “mild”; or (c) the presence of heartburn or regurgitation ≥ “about once per month” with associated severity level ≥ “severe.”13 Responses were recorded on two page No-Carbon-Required (NCR) paper. One copy was retained for data collection and the second copy was given to the patient. The questionnaire was reviewed by the clinical faculty and individuals indicating a “yes” response to heartburn or acid regurgitation were referred to their primary care provider for evaluation.
Survey responses referring to demographics were calculated using frequency percentiles and summary statistics. Differences in demographics across GERD diagnostic test results were assessed via global Fisher’s Exact Test.14 Using the known sensitivity and specificity parameters with 95% intervals of the GERD diagnostic test, adjusted prevalence estimates for actual GERD diagnosis with exact 95% confidence intervals were calculated.15,16 All statistical tests were performed at an alpha threshold of 0.05. All statistical analyses were performed in STATA® statistics/data analysis software version 11.2.
Results
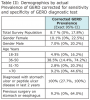
A total of 227 respondents successfully completed the GERD screening questionnaire. Using the diagnostic test reported in Offman et al. for testing positive (+) for GERD from the questionnaire answers, 20 respondents of the total 227 respondents tested positive (+) for GERD with 207 testing negative (-) as per the screening questionnaire.13 Table 2 shows summary statistics of demographics by GERD testing status.
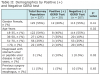
The majority of respondents were female (55%) and between the ages of 18-25 years of age (54%), with a small percentage of respondents reporting prior diagnosis of a stomach or peptic ulcer in the last 2 years (1%) and having previous surgery on their stomach or esophagus (5%). Results of the GERD test shows a trend of decreasing prevalence of a positive test (+) with increasing age (global Fishers Exact Test, P = .02). Additionally, respondents who reported a previous diagnosis of a stomach or peptic ulcer were more likely to test positive (+) for GERD as per the diagnostic questionnaire (P = .02). Using the previously reported sensitivity and specificity parameters of the GERD diagnostic test (sensitivity 61.4%, 95% CI: 49% to 72.8%; specificity 96.2%, 95% CI: 91.4% to 98.8%), Table 3 shows prevalence estimates adjusted for the imperfect diagnostic test, resulting in an estimate of the true prevalence estimates of GERD with Exact Binomial 95% confidence intervals.
The true prevalence of GERD in the study population was 8.7% (95% CI 0%, 17.8%). When only considering estimation of the point estimates, the prevalence of GERD among females was slightly higher (10.1%) than among males (7%), with age subgroup 36-50 years of age having the largest prevalence of GERD (38.5%), and subgroup 51-70 years of age having the lowest (2.8%). When the confidence intervals constructed around the point estimates are interpreted, as per adjustment for the imperfect diagnostic test, there were no statistically significant differences observed in demographics by actual prevalence of GERD.
Discussion
Twenty out of 227 respondents were identified as GERD sufferers. The GERD screening questionnaire used in this study was developed and validated to be utilized as a case-finding tool for patients with symptoms of GERD and was found to be a sufficient and accurate means to screen for GERD in a community dental setting.13 Practical, valid and reliable GERD screening questionnaires should be developed and routine screening should be implemented for oral healthcare providers, as oral cancer screening is.17
The findings are noteworthy because they demonstrate GERD screening can be implemented in a dental setting. Moreover, this study provides a template for how to effectively implement GERD screening in a dental setting to increase early detection and promote collaborative work among dental and medical professionals. Routine oral cancer screening is critical, but GERD symptoms screening is of equal importance. GERD symptoms can affect individuals’ quality of life and symptom complications can be fatal. Oral healthcare professionals’ utilization of a GERD symptoms questionnaire will increase awareness of GERD symptoms among patients at risk and potentially bridge the gap between dental and medical professionals. Collaboration among healthcare providers would represent a significant step toward increasing the awareness of GERD symptoms and promoting overall health.
Oral health professionals are the first line of defense in detecting oropharyngeal at an early stage. A GERD screening tool could be effective in recognizing the early symptoms of GERD in order to increase awareness and the management of the oral and systemic complications. Likewise, identifying GERD sufferers reporting increased frequency and duration of exposure to symptoms of heartburn and/or regurgitation may reduce the incidence of EAC, a highly lethal cancer with an increased incidence in the United States and Western Europe.12,18
Study limitations include a small population in only two locations, as well as the absence of follow-up among patients referred to their primary care providers for further evaluation of GERD.
Conclusion
This study explored routine assessment for GERD symptoms among dental patients and provided referrals to primary care providers of patients indicating GERD symptoms. To the authors’ knowledge, this is the first study to implement a GERD screening in a community dental setting. By way of completing the survey questionnaire, participants also gained awareness about GERD, enabling them to think about symptoms they may have otherwise overlooked. GERD symptoms are often not addressed because they are sometimes unseen or unnoticed.
Future studies should explore the development and implementation of a validated, reliable and practical combined risk assessment tool for oropharyngeal cancer and GERD, along with an efficient routine screening regimen, to promote early detection. Additionally, future studies should also include a follow-up mechanism for individuals referred to a primary care provider.
About the Author
Anisha Raibrown, RDH, MSDH is practicing clinician and the Director of the Boston Public Health Commission’s Office of Oral Health, Boston, Massachusetts. Lori J. Giblin, RDH, MS, is an Associate Professor, Linda D. Boyd, RHD, RDH, EdD., is Professor and Dean, and Kristen Perry, RDH, MS, is an Assistant Professor, all at the Forsyth School of Dental Hygiene, MCPHS University, Boston, Massachusetts.
References
1. Ranjitkar S, Smales RJ, Kaidonis JA. Oral manifestations of gastroesophageal reflux disease. J Gastroenterol Hepatol. 2012;27(1):21-27.
2. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastrooesophageal reflux disease: A systematic review. Gut. 2014;63(6):871-880.
3. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
4. National Institute of Diabetes and Digestive and Kidney Diseases [Internet] Bethesda: 2016. Gastroesophageal reflux (GER) and gastroesophageal reflux disease (GERD); [cited 2016 Mar 1] http://www.niddk.nih.gov/healthinformation/health-topics/digestive-diseases/barretts-esophagus/Documents/barretts_508.pdf.
5. Merrouche M, Sabate JM, Jouet P, et al. Gastroesophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17(7):894-900.
6. Moraes-Filho JP, Navarro-Rodriguez T, Barbuti R, et al. Guidelines for the diagnosis and management of gastroesophageal reflux disease: An evidence-based consensus. Arq Gastroenterol. 2010;47(1):99-115.
7. Stanghellini V, Armstrong D, Monnikes H, Bardhan KD. Systematic review: Do we need a new gastrooesophageal reflux disease questionnaire? Digestion. 2007;75 suppl 1:3-16.
8. Di Fede O, Di Liberto C, Occhipinti G, et al. Oral manifestations in patients with gastro-oesophageal reflux disease: A single-center case-control study. J Oral Pathol Med. 2008;37(6):336-340.
9. National Institute of Diabetes and Digestive and Kidney Diseases [Internet] Bethesda: 2016. Barrett’s esophagus; [cited 2016 Mar 1] http://www.niddk.nih.gov/healthinformation/health-topics/digestive-diseases/barretts-esophagus/Documents/barretts_508.pdf.
10. Absi A, Adelstein DJ, Rice T, eds. Esophageal cancer. Cleveland Clinic Center for Continuing Education [Internet] Cleveland (OH) Cleveland Clinic; 2010. [cited 2016 Mar 1] http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hematologyoncology/esophageal-cancer/.
11. Hoffman M, Haines CD. Esophageal cancer on the rise. [Internet] WebMD 2016 [cited 2016 Mar1] http://webmd.com.
12. Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett’s and esophageal adenocarcinoma consortium (BEACON). PLoS One. 2014;9(7):e103508.
13. Ofman JJ, Shaw M, Sadik K, et al. Identifying patients with gastroesophageal reflux disease: Validation of a practical screening tool. Dig Dis Sci. 2002;47(8):1863-1869.
14. Fisher RA. On the interpretation of Χ2 from contingency tables, and the calculation. Journal of the Royal Statistical Society.1922;85(1):87-94.
15. Lang Z, Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev Vet Med. 2014;113(1):13-22.
16. Reiczigel J, Foldi J, Ozsvari L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010;138(11):1674-1678.
17. Oral Cancer Foundation [Internet]. Newport Beach: 2016. Cancer screening protocols; 2016 [cited 2016 Mar 1] http://oralcancerfoundation.org.
18. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-713.