You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Dental clinicians play a vital role in their patients’ oral and systemic health. Research indicates that patients are 24.1% more likely to visit the dentist as compared with an annual exam by a physician; thus, dental health professionals are in a position to be the first providers to detect systemic health conditions.1 Dental hygienists play a crucial role in detecting disease and health risks and are able to communicate findings to their patients. Therefore, it is essential that dental hygienists are knowledgeable about systemic and oral health conditions enabling them to discuss risks, characteristics of diseases, medical referrals and treatment options.
Obstructive sleep apnea (OSA) is a health condition for which the dental community can identify and screen. However, less than 50% of dentists are actually capable of identifying the signs and symptoms of this sleep disorder.2 OSA is the most common breathing disturbance,3 with up to 80% of moderate-to-severe OSA remaining undiagnosed.4 This sleep disorder is defined as repeated episodes of complete or partial obstruction of the upper airway during sleep and characterized by snoring, witnessed stopped breathing, and excessive daytime sleepiness.3-4 OSA is associated with many health conditions such as hypertension,4-7 reduced quality of life,4-7 and diabetes mellitus.2,5 There is also a significant association between OSA and moderate-to-severe periodontitis.8-10 This correlation suggests that optimum oral health is imperative, especially when there is a risk for OSA.
OSA is important to dentistry because dental professionals have the opportunity to screen for risk factors and disease patterns of OSA and have the ability to assess one’s risk. There are several OSA detection tools available that determine the risk of OSA. The purpose of this literature review is to identify the role of the dental hygienist in detecting the risk of OSA and to gain knowledge on the topic in order to educate patients.
Definition and Categories of Sleep Apnea
Sleep apnea is a medical disorder characterized by a 50% decrease in airflow (hypopnea) or the complete cessation of airflow (apnea) occurring longer than 10 seconds while sleeping.6,8 The apnea-hypopnea index (AHI) is the most common index for assessing the severity of sleep apnea by combining the number of apneas and hypopneas each night (Table 1).
There are three categories of sleep apnea: OSA, central sleep apnea, and complex sleep apnea. OSA is the occurrence of five or more events per night of apnea and hypopnea due to an obstruction of the airway.11 Central sleep apnea is defined as a decrease in oxygen saturation levels due to the brain failing to deliver a signal for the body to breathe, resulting in ineffective and shallow breaths.11 Complex sleep apnea is a combination of OSA and central sleep apnea.
OSA: Characteristics, Risk Factors, and Health-Related Conditions
OSA received clinical recognition in the 1980s and has gradually gained recognition in the research setting.12 OSA remains the most common undiagnosed sleep disorder and chronic disease in Western society.2 Recent research suggests that up to 80% of moderate to severe cases of OSA are underdiagnosed,4 and one in five adults have mild OSA and one in 15 have moderate OSA.13 Characteristics of OSA include snoring, daytime sleepiness, and witnessed stopped breathing during sleep. Other symptoms are the sensation of choking while sleeping, insomnia, and daytime tiredness. Oral manifestations include attrition and a large tongue, along with the physical characteristic of a neck larger than 17 cm.
Obesity is considered the greatest risk factor associated with OSA. Even a person with mild to moderate obesity is at an increased risk for having OSA.5 Also, people with a high body-mass index, large neck circumference, and a high waist-to-hip ratio are at risk for developing OSA.4,5 Due to excessive daytime sleepiness, individuals are at an increased risk of motor vehicle accidents when left untreated.14 Research suggests that African Americans have a two-times greater risk to have OSA than Caucasians.15 Men have a greater predilection4,5 for OSA, about two- to three-fold than women.16 However, menopause and pregnancy may be an exception due to physiological and hormonal changes.13 Other risk factors include alcohol consumption,4 smoking,4 nasal congestion,10 and upper airway anatomy.4
OSA is independently linked to and increases the likelihood of several health-related conditions such as hypertension,4-7 decreased quality of life,4,7,17 diabetes mellitus,2,5 stroke,2 and diminished neurocognitive function.4 Additionally, there is an increased risk of congestive heart failure, coronary artery disease, myocardial infarction, and cardiac arrhythmias.2
Recent research has found an increase in periodontitis in individuals with OSA8 as they share several risk factors such as male sex, older age, obesity, oral breathing, cigarette smoking, and alcohol consumption.8 OSA and periodontitis are both associated with systemic inflammation and cardiovascular risk. Gunaratnam et al suggests, “There is a significant association between periodontal clinical attachment level and total sleep time.”9 Furthermore, there is a positive significant association between OSA and periodontitis.8,10
Diagnosing OSA—Polysomnography: The Gold Standard
Polysomnography is considered the gold standard of diagnosing OSA and is completed in a laboratory. This diagnostic tool measures an individual’s sleep cycles and stages, recording air flow in and out of the lungs, blood oxygen levels, brain waves, eye movement, and heart rate.18 The disadvantages of polysomnography are low availability, cost, and time consumption.3,7 Because of the disadvantages, screening tools have been developed that may assist in identifying high-risk individuals. These tools may help serve the population by alerting those who are at risk, allowing them the opportunity to seek out definitive diagnosis.
Screening Tools for Detecting the Risk of Obstructive Sleep Apnea
Although physicians are the only professionals licensed to diagnose OSA, dentists and dental hygienists can detect OSA risk through screening tools. Some of these tools are the Mallampati classification,19-21 Epworth Sleepiness Scale,22,23 STOP Questionnaire,3 STOP-Bang Questionnaire,24 and ARES Questionnaire.2
Mallampati Classification
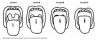
The Mallampati classification is an evaluation predicting the ease of intubation and is higher in people who show severe degrees of OSA.19,20 With the patient sitting upright, mouth open, and tongue protruded, the evaluator classifies the visibility of the posterior pharynx with aid of a light with a scale of I, II, III, and IV (Table 2, Figure 1).21 A score of I and II are considered adequate exposure of the posterior pharynx, while a score of III and IV are considered inadequate exposure of the posterior pharynx.20 Kandray et al assessed the inter-rater reliability of using the Mallampati classification between dental hygiene students and a supervising dentist to evaluate and classify the pharyngeal soft tissues.19 After training by a licensed respiratory therapist, 21 dental hygiene students received a diagram with the Mallampati classification and placed a check mark beside the image that best represented the patient’s oropharynx opening during a dental hygiene appointment. Subsequently, the dentist did an independent evaluation of each patient using the same type of diagram that the student used after the same training. The results show a 77% agreement between the dentist and students, indicating that dental hygiene students can classify oropharyngeal tissues.19
Epworth Sleepiness Scale
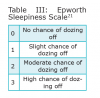
The Epworth Sleepiness Scale (ESS), developed by Murray Johns in 1991, is a self-administered questionnaire that evaluates sleep tendencies.23 An individual rates the chance of dozing off in 8 different scenarios in this subjective questionnaire (Table 3 and Table 4).22 An ESS score of 16 or greater is considered a high level of daytime sleepiness, which is one of the most common symptoms for OSA.17 Nguyen et al conducted a study to evaluate the reproducibility of the ESS.22 In this study, 142 patients were evaluated, and it was found that there were discrepancies when the ESS was repeated over time in the same untreated individual. The researchers cautioned that the ESS should not be the sole tool to indicate risk for possible OSA due to reproducibility discrepencies.22
STOP Questionnaire
Chung et al constructed a concise screening tool for OSA called the STOP questionnaire (Table 5).3 The initial goal was to find a tool for identifying OSA risk in patients soon undergoing surgical procedures; recently it has begun to identify any patient at risk. If a patient answers “yes” to two or more of the questions, then that patient is considered at high risk.
Chung et al investigated 278 patients who answered 14 questions in order to identify the best questions to ask to identify OSA. This investigation culminated in the four-question STOP questionnaire.3 Then a pilot study was implemented on 592 preoperative clinic patients to ensure the strength of the STOP questionnaire. After the pilot study, the questionnaire was administered to 1,875 patients with a 100% completion rate. Then, regardless of the score, they were all asked to undergo an overnight polysomnographic. The AHI from the polysomnography was used against the STOP questionnaire in order to validate it. Two hundred and eleven patients agreed to the polysomnography (34 from the pilot study, 177 from the study). Overall, it was found that the STOP questionnaire had a moderately high sensitivity and positive predictive value, indicating that the STOP questionnaire is more sensitive in determining if one has moderate-to-severe OSA. The positive predictive value was significantly increased when people have the following clinical characteristics: male gender, age older than 50 years, BMI greater than 35 kg/m2, and neck circumference greater than 40 cm.3 The study concluded that the STOP questionnaire is an easy-to-use screening tool for patients at risk with OSA.3
Other studies have used the STOP questionnaire to determine OSA risk. Ahmad et al found that the STOP questionnaire aided in identifying one’s risk of OSA and confirmed that the prevalence of periodontitis may be higher in patients with OSA.8
STOP-Bang Questionnaire
The STOP-Bang questionnaire is a series of eight questions with scores ranging from 0 to 8 (Table 6).24 If an individual answers yes to three or more items, then that patient is considered at high risk for OSA. Research indicates that a STOP-Bang score of 3 or greater reveals a high sensitivity for moderate-to-severe OSA along with an increase in specificity and predicted probability.24
The Apnea Risk Evaluation System Questionnaire
The Apnea Risk Evaluation System (ARES) can sufficiently assess the risk of an individual with undiagnosed OSA.2 Levendowski et al assessed the prevalence of potential OSA in a dental population by administering this questionnaire to two dental practices.2 The first practice had no prior OSA treatment experience, and the questionnaire was given to the patients at a scheduled dental appointment with 229 patients fully completing the ARES questionnaire. The second practice had 15 years of prior experience with OSA treatment, and the questionnaire was mailed to 870 patients with 102 returning it fully completed. Including both practices, 102 patients were considered high risk upon completing the questionnaire and were selected to complete a 2-night sleep study. During the night, the patients wore a wireless unicorder on their foreheads that recorded oxygen saturation, pulse rate, airflow, respiratory effort, snoring levels, head movement, and head position. Results from the ARES questionnaire found that 67% of the men were at high risk of having at least mild OSA and 33% were at risk for having moderate-to-severe OSA. Twenty-eight percent of the women had at least mild OSA, and 6% had moderate-to-severe OSA. Out of the 105 high-risk patients participating in the sleep study, 96% had an AHI greater than five events per hour2 and 70% had an AHI of greater than 20 events per hour. In conclusion, the study found a high prevalence of undiagnosed sleep apnea in this dental population, providing additional evidence that the ARES can sufficiently assess the risk of an individual with undiagnosed OSA.
Treatment Options for Obstructive Sleep Apnea
Treatment of OSA includes lifestyle changes such as weight loss,25 upper airway surgery,25 continuous positive airway pressure (CPAP),25-27 and oral appliances.28,29
Continuous Positive Airway Pressure
CPAP is the gold standard treatment of OSA and is effective in decreasing the nocturnal events of OSA.25 Research suggests the therapeutic effects of being on CPAP for 3 weeks significantly reduces fatigue and increases energy in individuals with OSA.26 It has also been found that CPAP reduces cardiovascular risk, fatal or nonfatal cardiovascular events, and blood pressure within 3 weeks of complying with CPAP treatment.27 CPAP improves glucose control in diabetic patients suffering from severe OSA.6 However, many individuals have difficulty complying due being uncomfortable while sleeping and the changes in occlusion. Mehta et al6 conducted a follow-up study to Chung et al,24 by contacting the patients who participated in OSA treatment. Among the patients given CPAP therapy, 40 stated that they were compliant and had significant reductions in medications for comorbidities than the patients who were non-compliant.
Additionally, the patients who were compliant showed a significant improvement in snoring, sleep quality, and daytime sleepiness. Based on these results, the researchers concluded OSA symptoms and severity of comorbidities might be reduced by timely diagnosis and compliance to treatment.6
Oral Appliances
Oral appliances are typically used when a patient does not like CPAP, has not responded to CPAP, or is not appropriate for CPAP. The appliances are small, are easy to wear, and typically take only a few weeks for patients to adjust. Before receiving, the patient has an examination to determine which oral appliance is most appropriate, fit and adapt the appliance, and determine the function. Dentists construct and fit oral appliances.25 Oral appliances for OSA treatment include tongue-retaining devices and mandibular advancement appliances.28 Tongue-retaining devices are seldom used except when dental reasons prohibit making mandibular advancement appliances. The appliance holds the tongue in a forward position with a suction bulb that prevents the tongue from obstructing the airway. The most commonly used oral appliance is the mandibular advancement appliance. Mandibular advancement appliances work by protruding the mandible forward, which minimizes upper airway collapse while sleeping. Treatment with oral appliances is noninvasive and reversible,28 accompanied occasionally with initial side effects of tooth pain, temporomandibular joint pain, dry mouth, gum irritation, and excessive salivation.29
Discussion
While dentists and dental hygienists cannot diagnose OSA, they have the opportunity to play a crucial role in the detection, education, medical referral, and treatment in OSA. Research for OSA is emerging and revealing a role for screening and treatment in dentistry. Because patients are more likely to visit their dentists than physicians in a year, dental hygienists and dentists can play an integral role in the detection of OSA by screening and referring high-risk patients to physicians. This can provide patients with interprofessional care by facilitating aid in OSA treatment from the dentist, dental hygienist, and physician. Collaboration with dentistry and medicine is pivotal because it establishes a team between the disciplines and provides patients with the best standard of care.
Dental hygienists can use screening tools, like the Mallampati classification, STOP questionnaire, and STOP-Bang questionnaire, which provide an opportunity to assist in detection. Research studies suggest dental hygienists can effectively use these tools in an appointment. While screening tools are available, these tools need further research on their reliability and validity to provide further proof of the effectiveness. As stated in Nguyen et al, more research is needed to evaluate the reproducibility of the ESS.24
As emerging research suggests a relationship between OSA and periodontal disease, OSA screening may become more important for periodontal risk assessment. Current studies have indicated that OSA may increase periodontitis presence by contributing to increased systemic inflammation. Dental hygienists are in an optimum position to educate patients on periodontitis and ways to arrest the condition (ie, OSA) through oral hygiene instructions. Further research is needed to determine the causal relationship between OSA and periodontitis.
Most dental school curricula do not provide adequate content for sleep apnea.30 It is imperative that sleep disorder education is presented in the curriculum of dental and dental hygiene programs as research has suggested that there are screening tools that can effectively detect OSA. After diagnosis, the dental team can aid in the patient’s treatment of OSA and play a crucial role in helping people who suffer from it. Furthermore, patients typically value what their dental hygienists say; therefore, it is important that hygienists receive education pertaining to sleep disorders, so the patient receives evidence-based facts regarding this disorder.
Conclusion
Dental hygienists are at a pivotal position to assist the interprofessional team in screening for OSA. As patients are more likely to visit their dentists regularly compared with physicians, more opportunities arise for screening and detection of systemic diseases, including OSA. The tools used to detect OSA are easily implemented during the evaluation or during the intraoral examination performed. Dental hygienists have the opportunity to educate the patient on risk factors, potential health conditions, and treatment options for OSA. Medical referrals are also part of the dental hygienist role, initiating the collaboration between the medical and dental fields. By including the dental team in screening and detection for OSA, patients may be more likely to receive accurate diagnosis and seek treatment, thus improving their overall quality of life.
About the Authors
Elizabeth C. Kornegay, RDH, BSDH, was a Bachelor of Science Degree candidate at the University of North Carolina at Chapel Hill (UNC) at the time of this submission. She is was also a second-year graduate student at UNC in the Dental Hygiene Education Master of Science Degree Program. Jennifer L. Brame, RDH, MS, is a Clinical Assistant Professor in the Department of Dental Ecology at UNC School of Dentistry.
References
1. Strauss SM, Alfano MC, Shelley D, Fulmer T. Identifying unaddressed systemic health conditions at dental visits: patients who visited dental practices but not general health care providers in 2008. Am J Public Health. 2012;102(2):253-255.
2. Levendowski DJ, Morgan T, Montague J, et al. Prevalence of probable obstructive sleep apnea risk and severity in a population of dental patients. Sleep Breath. 2008;12:303-309.
3. Chung F, Yegneswaran B, Lia P, et al. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812- 821.
4. Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349-364.
5. Schwartz AR, Patil SP, Laffan AM, et al. Obesity and obstructive sleep apnea. Am Thoracic Soci. 2008;5:185-192.
6. Mehta V, Subramanyam R, Shapiro CM, Chung F. Health effects of identifying patients with undiagnosed obstructive sleep apnea in the preoperative clinic: a follow-up study. J Can Anesthes. 2012;59:544-555.
7. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. J Can Anesthes. 2010;57:423-438.
8. Ahmad NE, Sanders AE, Sheats R, et al. Obstructive sleep apnea in association with periodontitis: a case-control study. J Dent Hyg. 2013;87(4):188-199.
9. Gunaratnam K, Taylor B, Curtis B, Cistulli P. Obstructive sleep apnea and periodontitis: a novel association? Sleep Breath. 2009;13:233-239.
10. Seo WH, Cho ER, Thomas RJ, et al. The association between periodontitis and obstructive sleep apnea: a preliminary study. J Periodont Res. 2012;48(4):500-506.
11. Swecker T, Schroeder J. Sleep apnea screening in the dental office. Dimensions. 2014;12(1):42-46.
12. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136-143.
13. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Resp Crit Care. 2002;165:1217-1239.
14. Kay GG, Feldman. Effects of armodafinil on simulated driving and self-report measures in obstructive sleep apnea patients prior to treatment with continuous positive airway pressure. J Clin Sleep Med. 2013;9(5):445-454.
15. Ancoli-Israel S, Klauber MR, Stepnowsky C, et al. Sleep-disordered breathing in African-American elderly. Am J Resp Crit Care. 1995;152(6):1946-1949.
16. Strohl KM, Redline S. Recognition of obstructive sleep apnea. Am J Resp Crit Care. 1996;154:279-289.
17. Li Y, Zhang J, Lei F, et al. Self-evaluated and close relative-evaluated Epworth Sleepiness Scale vs. multiple sleep latency test in patients with obstructive sleep apnea. J Clin Sleep Med. 2014;10(2):171-176.
18. Polysomnography MedlinePlus [Internet]. 2014 [cited 2014 March 2]. Available from: http://www.nlm. nih.gov/medlineplus/ency/article/003932.htm
19. Kandray DP, Juruaz D, Yacovone M, Chang GA. Inter-rater reliability of the Mallampati classification for patients in a dental hygiene clinic. J Dent Hyg. 2013;87(3):134-139.
20. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict tracheal intubation: a prospective study. Can Anesth Soc J. 1985;32(4):429-434.
21. Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29(7):903-908.
22.Nguyen ATD, Baltzan MA, Small D, et al. Clinical reproducibility of the Epworth Sleepiness Scale. J Clin Sleep Med. 2006;2(2):170-174.
23. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545.
24. Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Brit J Anaesth. 2012;108(5):768-775.
25. Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11(1):1-22.
26. Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34(1):121-126.
27. Litvin AY, Sukmarova ZN, Elfimova EM, et al. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc Health Risk Manag. 2013;9:229-235.
28. Oral Appliances. American Academy of Dental Sleep Medicine [Internet]. 2014 [cited 2014 March 5]. Available from: http://www.aadsm.org/oralappliances.aspx
29. Doff MH, Finnema KJ, Hoekema A, et al. Long-term oral appliance therapy in obstructive sleep apnea syndrome: a controlled study on dental side effects. Clin Oral Invest. 2013;17:475-482.
30. Barsh LI. The recognition and management of sleep-breathing disorders: a mandate for dentistry. Sleep Breath. 2009;13:1-2.