You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
In contrast to an accumulation of individual bacteria, a biofilm is a complex, communal, 3-dimensional arrangement of bacteria. Bacterial biofilms are ubiquitous and are potentially found in a variety of sites within the human body. For example, they can grow on indwelling catheters, ports, and implants; external surfaces of the eye; artificial heart valves; endotracheal tubes; and contaminated prosthetic joints. A bacterial biofilm is often the cause of persistent infections and has been associated with osteomyelitis, pneumonia in patients with cystic fibrosis, and prostatitis.1
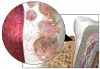
In areas related to oral health care, bacterial biofilms are found in dental unit water lines, on tooth surfaces and dental prosthetic appliances, and on oral mucous membranes. Biofilm in the form of supragingival and subgingival plaque is the etiologic agent in dental caries and periodontal diseases (Figure 1).2-5 The pathogenicity of the dental plaque biofilm is enhanced by the fact that in biofilm form, the component bacteria have increased resistance to antibiotics and other chemotherapeutic agents and are less able to be phagocytized by host inflammatory cells. Therefore, control of the dental plaque biofilm is a major objective of dental professionals and critical to the maintenance of optimal oral health. This article reviews the characteristics of dental biofilm, its role in the etiology of periodontal diseases, and strategies for controlling the biofilm to promote health.
Changing Views of Dental Plaque
Over the past 50 years, the understanding and characterization of dental plaque have undergone significant evolution. Loesche6 proposed both a non-specific and a specific plaque hypothesis for periodontal disease initiation and progression.
The nonspecific plaque hypothesis proposed that the entire microbial community of plaque that accumulated on tooth surfaces and in the gingival crevice contributed to the development of periodontal disease. Plaque bacteria produced virulence factors and noxious products that initiated inflammation, challenged the host defense system, and resulted in the destruction of periodontal tissues. Under this hypothesis, the quantity of plaque was considered to be the critical factor in the development of periodontal disease. Thus, increases in the amount of plaque (quantity), as opposed to specific pathogenic microorganisms (quality) found in the plaque, were viewed as being primarily responsible for inducing disease and disease progression.7,8
Studies on the microbial etiology of various forms of periodontitis support the specific plaque hypothesis, which proposes that only certain microorganisms within the plaque complex are pathogenic. Despite the presence of hundreds of species of microorganisms in periodontal pockets, fewer than 20 are routinely found in increased proportions at periodontally diseased sites. These specific virulent bacterial species activate the host's immune and inflammatory responses that then cause bone and soft tissue destruction.6,8,9
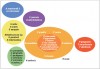
Socransky and colleagues4,10 recognized that early plaque consists predominantly of gram-positive organisms and that if the plaque is left undisturbed it undergoes a process of maturation resulting in a more complex and predominantly gram-negative flora. These investigators assigned the organisms of the subgingival microbiota into groups, or complexes, based on their association with health and various disease severities (Figure 2).4,10 Color designations were used to denote the association of particular bacterial complexes with periodontal infections. The blue, yellow, green, and purple complexes designate early colonizers of the subgingival flora. Orange and red complexes reflect late colonizers associated with mature subgingival plaque. Certain bacterial complexes are associated with health or disease.10,11 For example, the bacteria in the red complex are more likely to be associated with clinical indicators of periodontal disease such as periodontal pocketing and clinical attachment loss.
Plaque Recognized as a Biofilm
Research over the past decade has led to the recognition of dental plaque as a biofilm—a highly organized accumulation of microbial communities attached to an environmental surface. Biofilms are organized to maximize energy, spatial arrangements, communication, and continuity of the community of microorganisms.
Biofilms protect bacteria living within their structures and thereby provide an advantage over free-floating (planktonic) bacteria. The slimy extracellular matrix produced by biofilm bacteria encloses the microbial community and protects it from the surrounding environment, including attacks from chemotherapeutic agents. Chemotherapeutic agents have difficulty penetrating the polysaccharide matrix to reach and affect the microorganisms.1,11-13 Thus, the matrix helps to protect bacteria deep within the biofilm from antibiotics and antiseptics, increasing the likelihood of the colonies' survival. Furthermore, the extracellular matrix keeps the bacteria banded together, so they are not flushed away by the action of saliva and gingival crevicular fluid. Mechanical methods, including toothbrushing, interdental cleaning, and professional scaling procedures, are required to regularly and effectively disrupt and remove the plaque biofilm. Antiseptics, such as mouthrinses, can help to control the biofilm but must be formulated so as to be able to penetrate the plaque matrix and gain access to the pathogenic bacteria.
Biofilms have a definite architectural structure. The bacteria are not uniformly distributed throughout the biofilm; rather, there are aggregates of microcolonies that vary in shape and size. Channels between the colonies allow for circulation of nutrients and by-products and provide a system to eliminate wastes.14,15 Microorganisms on the outer surface of biofilms are not as strongly attached within the matrix and tend to grow faster than those bacteria deeper within the biofilm. Surface microorganisms are more susceptible to detachment, a characteristic that facilitates travel to form new biofilm colonies on nearby oral structures and tissues.
Bacteria in biofilm communicate with each other by a process called quorum sensing. This dynamic, sophisticated communication system enables bacteria to monitor each other's presence and to modulate their gene expression in response to the number of bacteria in a given area of the biofilm.8 In addition, as a result of quorum sensing, portions of the biofilm can become detached in order to maintain a cell density compatible with continued survival.
Stages of Biofilm Formation
The growth and development of biofilm are characterized by 4 stages: initial adherence, lag phase, rapid growth, and steady state. Biofilm formation begins with the adherence of bacteria to a tooth surface, followed by a lag phase in which changes in genetic expression (phenotypic shifts) occur. A period of rapid growth then occurs, and an exopolysaccharide matrix is produced. During the steady state, the biofilm reaches growth equilibrium. Surface detachment and sloughing occur, and new bacteria are acquired.
Initial Adherence and Lag Phase
The first phase of supragingival biofilm formation is the deposition of salivary components, known as acquired pellicle, on tooth surfaces. This pellicle makes the surface receptive to colonization by specific bacteria. Salivary glands produce a variety of proteins and peptides that further contribute to biofilm formation. For example, salivary mucins, such as MUC5B and MUC7, contribute to the formation of acquired pellicle,16,17 and statherin, a salivary acidic phosphoprotein, and proline-rich proteins promote bacterial adhesion to tooth surfaces.18 Acquired pellicle formation begins within minutes of a professional prophylaxis; within 1 hour, microorganisms attach to the pellicle. Usually, gram-positive cocci are the first microorganisms to colonize the teeth. As bacteria shift from planktonic to sessile life, a phenotypic change in the bacteria occurs requiring significant genetic up-regulation (gene signaling that promotes this shift). As genetic expression shifts, there is a lag in bacterial growth.
Rapid Growth
During the rapid growth stage, adherent bacteria secrete large amounts of water-insoluble extracellular polysaccharides to form the biofilm matrix. The growth of microcolonies within the matrix occurs. With time, additional varieties of bacteria adhere to the early colonizers—a process known as coaggregation—and the bacterial complexity of the biofilm increases. These processes involve unique, selective molecular interactions leading to structural stratification within the biofilm. Coaggregation and subsequent cell division also increase the thickness of biofilm.19-21
Steady State/Detachment
During the steady state phase, bacteria in the interior of biofilms slow their growth or become static. Bacteria deep within the biofilm show signs of death with disrupted bacterial cells and other cells devoid of cytoplasm; bacteria near the surface remain intact. During this phase, crystals can be observed in the interbacterial matrix that may represent initial calculus mineralization.22 As noted above, during the steady state stage, surface detachment and sloughing also occur, with some bacteria traveling to form new biofilm colonies.
Biofilm and Oral Disease
Biofilms can cover surfaces throughout the oral cavity. Microcolonies exist on oral mucosa, the tongue, biomaterials used for restorations and dental appliances, and tooth surfaces above and below the gingival margin (Figure 3). It is important for oral health professionals to communicate to their patients that both dental caries and periodontal disease are infectious diseases resulting from dental plaque biofilm accumulation. Each of these diseases requires specific strategies for prevention and treatment.
With respect to periodontal disease, dental plaque biofilm demonstrates a succession of microbial colonization with changes in bacterial flora observed from health to disease. Researchers studied over 13,000 plaque samples from 185 patients with conditions ranging from oral health to periodontal disease.4,23 As noted above, based on their findings, a number of microbial complexes were identified that were associated with various stages of disease initiation and progression. Bacterial species contained in the yellow, green, and purple complexes appear to colonize the subgingival sulcus first and predominate in gingival health. In contrast, orange complex bacteria are associated with gingivitis and gingival bleeding. Interestingly, bacteria of the orange complex may also be associated with red complex microorganisms including Porphyromonas gingivalis, Tannerella forsythensis, and Treponema denticola, organisms found in greater numbers in diseased sites and in more advanced periodontal disease.10,24
Bacterial communities living in a biofilm possess resourceful survival strategies, including a broader habitat for growth, nutrition, waste elimination, and new colonization; environmental niches for safety; barriers to thwart antimicrobial drug therapy; protection from the host's defense system including phagocytosis; and enhanced pathogenicity.1,8 These strategies account for the ongoing challenge of successfully controlling periodontal infection and disease progression.25
As the biofilm matures and proliferates, soluble compounds produced by pathogenic bacteria penetrate the sulcular epithelium. These compounds stimulate host cells to produce chemical mediators associated with the inflammatory process.26
- Interleukin-1 beta (IL-1β), prostaglandins, tumor necrosis factor alpha (TNF-α), and matrix metalloproteinases are mediators that recruit neutrophils to the area via chemotaxis and cause increased permeability of gingival blood vessels, permitting plasma proteins to migrate from within the blood vessels into the tissue.
- As the gingival inflammatory process continues, additional mediators are produced, and more inflammatory cell types such as neutrophils, T cells, and monocytes are recruited to the area.
- Proinflammatory cytokines are produced in the tissues as a response to the chronic inflammatory process, and these proteins may further escalate the local inflammatory response and affect the initiation and progression of systemic inflammation and disease.
The result of this chronic inflammation is a breakdown of gingival collagen and accumulation of an inflammatory infiltrate, leading to the clinical signs of gingivitis. In some individuals, the inflammatory process will also lead to the breakdown of collagen in the periodontal ligament and resorption of the supporting alveolar bone. It is at this point that the lesion progresses from gingivitis to periodontitis, continuing the same challenge from proinflammatory mediators as with chronic gingivitis. Thus, controlling dental plaque biofilm is essential to preventing and reversing gingivitis as well as preventing and managing periodontitis.
Periodontal Biofilm Infection and Systemic Health
In recent years, studies have demonstrated an association between periodontitis and various systemic diseases and conditions, including cardiovascular disease, diabetes mellitus, respiratory disease, adverse pregnancy outcomes, obesity, pancreatic cancer, and Alzheimer's disease.27-57 While several of these associations have not been definitively established, biological mechanisms explaining some of the more extensively studied relation- ships are emerging.
The association between periodontal disease and some systemic diseases may relate to the ability of subgingival plaque bacteria and/or their products to gain access to the systemic circulation through the ulcerated epithelium of the periodontal pocket. For example, environmental niches like a subgingival pocket that contains anaerobic gram-negative microorganisms can potentially seed orange and red complex bacteria and/or their products to distant sites through the circulatory system. In this way, a dental biofilm infection can potentially contribute to both oral and systemic inflammation.25
Research on Periodontal Microorganisms
Atheromas. Direct evidence for the role of dental biofilm infection in systemic inflammation comes from findings of periodontal microorganisms in human carotid atheromas. Studies of atheromatous lesions in carotid arteries revealed that over 40% of atheromas contain antigens from periodontal pathogens including P gingivalis, T forsythensis, and Prevotella intermedia.28,58 In addition, P gingivalis is known to induce platelet aggregation, a component of atheroma and thrombus formation,29 and invade endothelial cells in cell cultures.59 While such findings suggest a possible invasion of atheromas by oral pathogens as well as possible contribution to their development, it is important to note that causality has yet to be established.
Preterm Birth. Research suggests that periodontal pathogens may travel via the bloodstream from the oral cavity to the placenta initiating preterm birth. In an animal model, Han and coworkers60 found that periodontal bacteria, including Fusobacterium nucleatum, entered the bloodstream from ulcerated gingival sulci or periodontal pockets and negatively influenced the normal birth process.
Respiratory Disease. Likewise, biofilm in the oral cavity may serve as a reservoir of infection leading to respiratory disease. Pseudomonas aeruginosa, Staphylococcus aureus, and enteric bacteria have been shown to colonize the teeth of patients admitted to hospitals and long-term care facilities. These bacteria may be released into saliva and aspirated into the lower airway causing respiratory infection.46-49,61 Intubation is another vehicle by which bacteria from the oral biofilm can be directly introduced into the respiratory system. Intubation tubes support biofilm growth contributing to nosocomial infection such as pneumonia. This is one reason why oral intubation raises the risk of nosocomial infection in intensive and critical care hospital populations.
Association With Chronic Diseases and Conditions
Research has also suggested that the association between oral inflammation and systemic inflammation may be key to understanding and managing the significant, deleterious effects on the multiple organ systems involved in some chronic diseases and conditions (Figure 4).26
Cardiovascular Disease. Cardiovascular disease is characterized by inflammatory plaque accumulation in blood vessels that can cause thromboses and lead to myocardial infarction. Atherosclerosis represents a chronic inflammatory process that causes endothelial dysfunction and injury to the elastic and muscular arterial tissue. Early atherosclerotic lesions contain neutrophils, monocytes, and lymphocytes. These leukocytes can affect the vascular endothelial lining and cause oxidation of low-density lipoproteins. As a result, monocytes, induced to become macrophages, take up these oxidized lipoproteins and become lipid-laden foam cells. As the lesion progresses, the extracellular matrix of the vessel wall is degraded by proteolytic enzymes and becomes susceptible to rupture. Thromboses can occlude blood flow to the heart and brain and eventually lead to infarction, heart attack, or stroke.26
Since atherosclerosis is inflammatory by nature, identifying inflammatory markers that correlate with disease state is important. One recognized and consistent marker of systemic inflammation and poor cardiovascular prognosis is the acute-phase protein C-reactive protein (CRP), the level of which rises with systemic inflammation.62,63 Animal model studies of the relationship between cardiovascular disease and periodontal disease demonstrate that clinically induced oral infection with P gingivalis will increase atheroma size and elevate CRP levels in the blood.30 Conversely, some studies have shown that treatment of periodontitis decreases CRP blood levels,64 though this has not been a consistent finding.
Diabetes Mellitus. Diabetes mellitus is another chronic systemic disease associated with periodontitis. In fact, periodontitis has been identified as one of the major complications of diabetes.65 Although diabetes increases the susceptibility to periodontal disease,38,39,65 periodontitis may also increase the difficulty of maintaining satisfactory glycemic control in people with diabetes as compared with those with diabetes without periodontitis.40 One biological mechanism proposed to explain the increased incidence and severity of periodontal disease in individuals with diabetes is the finding of elevated levels of inflammatory mediators in the gingival crevicular fluid from periodontal pockets of patients with diabetes with poor glycemic control as compared with those with diabetes who are well controlled or those without diabetes. Those with poor glycemic control had considerable periodontal destruction with an equivalent bacterial challenge.39,66 Of note, the proinflammatory cytokine TNF-α plays a significant role in this process. TNF-α has a major role in insulin resistance, the primary cause of type 2 diabetes, and is produced in large quantities by fat cells. Periodontitis also has been associated with increased levels of TNF-α. Elevated levels of TNF-α may lead to greater bone loss by killing cells that repair damaged connective tissue or bone. Elevated TNF-α levels also may exacerbate insulin resistance and worsen glycemic control.44,66,67
Adverse Pregnancy Outcomes. Studies also demonstrate that periodontal diseases are associated with the risk of adverse pregnancy outcomes, especially preterm low-birth-weight infants.50-52 Chronic infection, such as that found with chronic periodontitis, can stimulate the inflammatory process throughout the body. In the placenta, this may lead to elevated amniotic levels of prostaglandins, TNF-α, and IL-1 and IL-6, stimulating premature rupture of membranes, preterm labor, and the birth of low-birth-weight infants. Intervention studies are currently under way to investigate a cause and effect relationship between advanced periodontitis and adverse pregnancy outcomes.
Strategies for Managing Dental Biofilm to Promote Health
Although dental biofilm cannot be completely eliminated, its pathogenicity can be lessened through effective oral hygiene measures. Daily toothbrushing, interdental cleaning, and the use of topical antimicrobial chemotherapeutics are patient-based strategies to reduce the bacterial biofilm and to help prevent periodontal diseases. American Dental Association (ADA)–Accepted antimicrobial mouthrinses have been shown to help prevent and reduce plaque and gingivitis when added to a daily oral hygiene regimen of mechanical plaque removal. Further, bacteria from the biofilm on mucosal and tooth surfaces are shed constantly into saliva and transferred to other areas of the mouth. Since oral mucosa, which represents about 80% of the oral cavity surface,68 can serve as a reservoir for pathogenic bacteria that can be transferred to the tooth surface and sulcus, supplementing mechanical plaque control methods with topical antimicrobials may also play an important role in reducing reservoirs of pathogens that are unaffected by brushing and flossing directed at the tooth surface.
Using Evidence in Practice
Products recommended to patients should be those that have documented efficacy and safety. Only 2 nationally branded antiseptic mouthrinses and their generic equivalents have received the ADA Council on Scientific Affairs Seal of Acceptance for control of supragingival plaque and gingivitis: Listerine® (fixed combination of essential oils) and Peridex® (0.12% chlorhexidine gluconate). However, due to recent changes in the ADA Seal Program, Peridex® and its generic equivalents no longer carry the ADA Seal because chlorhexidine gluconate is a prescription product. The fixed combination of essential oils and cetylpyridinium chloride have also been reviewed by a Food and Drug Administration (FDA) advisory committee and have received a Category I recommendation, meaning they have been found to be safe and effective for the control of supragingival plaque and gingivitis. Peridex® and its generic equivalents, which are prescription products, have been approved for marketing by the FDA via the New Drug Application route (or for generics, the Abbreviated New Drug Application process). Examples of effective antimicrobial mouthrinses currently on the market appear in Table I.
Conclusion
Dental biofilm is a complex, organized microbial community that is the primary etiologic factor for the most frequently occurring oral diseases, dental caries and periodontal diseases. Although the dental biofilm cannot be eliminated, it can be controlled with comprehensive mechanical and chemotherapeutic oral hygiene practices. Teaching patients to use daily brushing, interdental cleaning, and antimicrobial mouthrinses that carry the ADA Seal of Acceptance increases the likelihood of periodontal disease prevention and reduction. Although additional research is needed, there is the possibility that these cost-effective, preventive strategies may minimize the effect of periodontal diseases on specific systemic conditions.
References
1. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318-1322.
2. van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672-681.
3. Stenudd C, Nordlund A, Ryberg M, et al. The association of bacterial adhesion with dental caries. J Dent Res. 2001;80:2005-2010.
4. Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144.
5. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111.
6. Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65-107.
7. Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal disease. J Clin Periodontol. 1986;13:905-911.
8. Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006;137(11 suppl):10S-15S.
9. Loesche WJ. DNA probe and enzyme analysis in periodontal diagnostics. J Periodontol. 1992;63:1102-1109.
10. Socransky SS, Haffajee AD. Periodontal microbial etiology. Periodontol 2000. 2005;38:135-187.
11. Socransky SS, Haffajee AD. Dental biofilms: difficult thera peutic targets. Periodontol 2000. 2002;28:12-55.
12. Brown MR, Gilbert P. Sensitivity of biofilms to antimicrobial agents. J Appl Bacteriol. 1993;74(suppl):87S-97S.
13. Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160-167.
14. Costerton JW, Lewandowski Z, DeBeer D, et al. Biofilms, the customized microniche. J Bacteriol. 1994;176:2137-2142.
15. Wood SR, Kirkham J, Marsh PD, et al. Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J Dent Res. 2000; 79:21-27.
16. Levine MJ, Reddy MS, Tabak LA, et al. Structural aspects of salivary glycoproteins. J Dent Res. 1987;66:436-441.
17. Tabak LA, Levine MJ, Mandel ID, Ellison SA. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982;11:1-17.
18. Gibbons RJ, Hay DI. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439-445.
19. Costerton JW, Cheng KJ, Geesey GG, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435-464.
20. Gibbons RJ. Microbial ecology: adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984;63:378-385.
21. Whittaker CJ, Klier CM, Kolenbrander PE. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513-552.
22. Wirthlin MR Jr, Armitage GC. Dental plaque and calculus: microbial biofilms and periodontal diseases. In: Rose LF, Mealey BL, Genco RJ, Cohen W, eds. Periodontics: Medicine, Surgery and Implants. St. Louis, MO: Elsevier Mosby; 2004.
23. Socransky SS, Haffajee AD, Ximenez-Fyvie LA, et al. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontol 2000. 1999;20:341-362.
24. Kojima T, Yasui S, Ishikawa I. Distribution of Porphyromonas gingivalis in adult periodontitis patients. J Periodontol. 1993;64:1231-1237.
25. Grossi S, Mealey BL, Rose LF. Effects of periodontal infection on the systemic condition. In: Rose LF, Mealey BL, Genco RJ, Cohen W, eds. Periodontics: Medicine, Surgery and Implants. St. Louis, MO: Elsevier Mosby; 2004.
26. Gurenlian JR. Inflammation: the relationship between oral health and systemic disease. Access. 2006;20(4)(suppl):1-9.
27. Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res. 2002;90:2-4.
28. Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554-1560.
29. Herzberg MC, Meyer MW. Effects of oral flora on platelets: possible consequences in cardiovascular disease. J Periodontol. 1996;67:1138-1142.
30. Paquette DW. The periodontal-cardiovascular link. Compend Contin Educ Dent. 2004;25:681-692.
31. Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intima-media thickness: the oral infections and vascular disease epidemiology study (INVEST). Circulation. 2005;111:576-582.
32. Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. 2005;150:11-18.
33. Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15:403-413.
34. Chun YH, Chun KR, Olguin D, Wang HL. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. 2005;40:87-95.
35. Hung HC, Willett W, Merchant A, et al. Oral health and peripheral arterial disease. Circulation. 2003;107:1152-1157.
36. Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160:2749-2755.
37. Joshipura KJ, Hung HC, Rimm EB, et al. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke.2003;34:47-52.
38. Nishimura F, Takahashi K, Kurihara M, et al. Periodontal disease as a complication of diabetes mellitus. Ann Periodontol. 1998;3:20-29.
39. Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134:34S-40S.
40. Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67(suppl 10):1085-1093.
41. Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713-719.
42. Miller LS, Manwell MA, Newbold D, et al. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843-848.
43. Mealey BL, Rethman MP. Periodontal disease and diabetes mellitus: bidirectional relationship. Dent Today. 2003;22:107-113.
44. Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51-61.
45. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99-112.
46. Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793-802.
47. Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease: a systematic review. Ann Periodontol. 2003;8:54-69.
48. Hayes C, Sparrow D, Cohen M, et al. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol.1998;3:257-261.
49. Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72:50-56.
50. Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67(suppl 10):1103-1113.
51. Jeffcoat MK, Geurs NC, Reddy MS, et al. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. 2001;132:875-880.
52. Scannapieco FA, Bush RB, Paju S. Periodontal disease as a risk factor for adverse pregnancy outcomes: a systematic review. Ann Periodontol. 2003;8:70-78.
53. Stein PS, Scheff S, Dawson DR III. Alzheimer's disease and periodontal disease: mechanisms underlying a potential bi-directional relationship. Grand Rounds Oral-Sys Med. 2006;1:14-24D.
54. Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171-175.
55. Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 2003;78:176-181.
56. Al-Zahrani MS, Bissada NF, Borawskit EA. Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol. 2003;74:610-615.
57. Reeves AF, Rees JM, Schiff M, Hujoel P. Total body weight and waist circumference associated with chronic periodontitis among adolescents in the United States. Arch Pediatr Adolesc Med. 2006;160:894-899.
58. Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138:S534-S536.
59. Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;187:139-144.
60. Han YW, Redline RW, Li M, et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272-2279.
61. Scannapieco FA. Periodontal inflammation: from gingivitis to systemic disease? Compend Contin Educ Dent. 2004;25(suppl 1):16-25.
62. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836-843.
63. Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417-424.
64. D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156-160.
65. Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334.
66. Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127-135.
67. Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105:1117-1124.
68. Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30:644-654.
About the Author
Joann R. Gurenlian, RDH, PhD, is a former chair of the Department of Dental Hygiene at Thomas Jefferson University in Philadelphia and past president of the American Dental Hygienists' Association. She continues to consult and to offer continuing education services in the health care field. She has authored over 100 articles, is the coauthor of The Medical History: Clinical Implications and Emergency Prevention in Dental Settings, and is the recipient of numerous awards, including the American Dental Hygienists' Association Distinguished Service Award. She is the vice president of the International Federation of Dental Hygienists and chairs a work group for the National Diabetes Education Program.