You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Periodontal diseases are a group of conditions that cause inflammation and destruction to the supporting structures of the teeth. These chronic oral infections are characterized by the presence of a biofilm matrix that adheres to the periodontal structures and serves as a reservoir for bacteria. Dental plaque biofilm is a complex structure of bacteria that is marked by the excretion of a protective and adhesive matrix.1 Within this matrix are gram-negative anaerobic and microaerophilic bacteria that colonize on the tooth structures, initiate the inflammatory process, and can lead to bone loss and the migration of the junctional epithelium, resulting in periodontal pocketing and periodontal disease. This bacterial insult can result in destruction of the periodontal tissues which precipitates a systemic inflammatory and immune response.2
For many years, it was believed that specific pathogenic bacteria found within dental plaque biofilm were solely responsible for periodontal diseases. While it is known that pathogenic bacteria are one facet of the disease process and are consistently present, it is not the only cause of periodontitis. The host response to the bacterial insult modulates the severity of the disease by activating the immune system to mediate the disease process. How well the host responds to the pathogenic bacteria modulates how the disease is initiated and progresses. This is evidenced by the fact that gingivitis does not always progress into periodontitis.
Over the years, several risk factors for periodontitis have been identified. For example, stress, poor dietary habits with high sugar intake, smoking and tobacco use, obesity, age, and poor dental hygiene all contribute to the development of periodontal disease. Other major risk factors include clinching or grinding teeth, genetic factors, other family factors, other medical diseases such as diabetes, cancer, or AIDS, defective dental restorations medication use, and conditions that change estrogen levels (puberty, pregnancy, menopause).3-4 Eighty percent of individuals with periodontal disease have at least one risk factor that increases their susceptibility to the infectious process and subsequent tissue damage. Often multiple factors are present.3-4
Initiative on Oral Health Care
The first-ever Surgeon General's Report on Oral Health in 2000 outlined the prevalence of oral diseases such as dental caries and periodontal infection. It also identified vulnerable populations that have a higher prevalence of oral disease, and that significant racial/ethnic and socioeconomic disparities exist in the United States. Subsequently, the surgeon general put forth a call for action to promote access to oral health care for all, reduce the morbidity of oral diseases, and eliminate oral health disparities. The report concluded that oral diseases can be associated with systemic conditions, including diabetes, heart disease, and adverse pregnancy outcomes. Specifically, the report stressed that periodontal treatment during pregnancy is an important strategy to potentially improve maternal and infant health.5
Oral health and its relationship to systemic health is important to society because up to 90% of the worldwide population is affected by periodontal disease—either gingivitis or periodontitis.6 Reports indicate that up to 30% of the general population has a genetic predisposition to periodontitis and a conservative estimate is that over 35 million people in the United States have periodontitis.7
Periodontal Disease and Other Systemic Conditions
There is considerable interest in the link between oral and systemic health among dental and medical providers. Current evidence suggests that periodontal disease is associated with an increased risk for cardiovascular disease,8,9 diabetes,10,11 community and hospital acquired respiratory infections,12 and adverse pregnancy outcomes.13-15 Individuals with periodontal disease have approximately a 1.5 – 1.9 increased odds for developing cardiovascular disease.8,16 There appears to be a bidirectional relationship between periodontal disease and diabetes with a 2- to 3-fold increased risk for diabetes among individuals with tooth loss. Teeth and periodontium may serve as a reservoir and may contribute to respiratory infections. Individuals with poor oral hygiene such as dental decay have a 2- to 9-fold increase odds for pneumonia.12 Many recent studies have reported that maternal periodontal disease may be an independent contributor to abnormal pregnancy outcomes including preterm birth, low birth weight, risk for preeclampsia, mortality, and growth restriction. However, the causality of how periodontitis influences pregnancy outcomes has not been established.14-25
Treatment of periodontal infection may reduce the risk of other systemic conditions. In a randomized clinical trial to estimate the effect of periodontal therapy on traditional and novel risk factors for cardiovascular disease and on markers of inflammation, D'Aiuto et al found that therapy reduced inflammatory cytokines, blood pressure, and cardiovascular risk scores.26 In a small treatment trial, type 2 diabetic patients showed improved diabetic control (lower HbA1c levels) after periodontal treatment.27 Several investigators have reported similar effects of oral health regimens on reduced risk for nosocomial respiratory infections. Treatment of mechanically ventilated patients with a daily oral hygiene regime consisting of an 0.12% chlorhexidine gluconate wash reduced the risk for nosocomial pneumonia.28,29 Recently, studies have been inconclusive on the effects of periodontal therapy during pregnancy for preventing adverse pregnancy outcomes.30-32 Treatment of oral infections may represent a novel approach to improving general health.
It is estimated that over 50% of pregnant women suffer from some form of gingival disease, either gingivitis or periodontitis,20,23 with the reports of prevalence fluctuating between 30%-100% for gingivitis and 5%-20% for periodontitis.33 The prevalence of periodontal diseases during pregnancy substantiates the strategy set forth by the surgeon general, in that periodontal treatment during pregnancy may potentially improve maternal and infant health.5
Pregnancy Complications
Maternal infections have long been recognized as increasing the risk for pregnancy complications such as preterm birth and preeclampsia. Preterm birth is delivery at less than 37 weeks gestation. Prematurity rates continue to increase. The latest statistics from the National Center for Health Statistics showed that for 2005 the preterm birth rate grew to 12.7%. This is up from 12.5% in 2004 and the preliminary reports for 2006 indicate an additional increase in the rates up to 12.8%. Since 1990, the rate of preterm birth has increased more than 20%.34
Understanding prematurity is important because it is the leading cause of death in the first month, causing up to 70% of all perinatal deaths.35 Even late premature infants, those born between 34 and 36 6⁄7 weeks gestation,36 have a greater risk of feeding difficulties, thermal instability, respiratory distress syndrome, jaundice, and delayed brain development.34 Prematurity is responsible for almost 50% of all neurological complications in newborns, and leads to lifelong complications in health, including but not limited to visual problems, developmental delays, gross and fine motor delays, deafness, and poor coping skills. These complications increase the health care dollars spent on each child. On average, the medical cost alone for a preterm birth is 10 times greater than the medical costs for a full-term birth. In 2005, the nationwide cost of preterm birth was more than $26.2 billion for health care, educational costs, and lost productivity.34 Although there have been advances in technology to help save the infants who are born premature or low birth weight, the lifelong problems associated with these conditions have not been abated.
Periodontal Disease and Its Impact on Pregnancy
Periodontal infection is one of many infections that have been associated with adverse pregnancy outcomes. The hypothesis that periodontal conditions influence the outcome of a pregnancy is not a new idea. In 1931, Galloway identified that the focal infection found in teeth, tonsils, sinuses, and kidneys pose a risk to the developing fetus. His information dated back to 1916 when pregnant guinea pigs were inoculated with streptococci eluted from human stillborn fetuses. This inoculation resulted in a 100% abortion rate. To show the impact on humans, he obtained a full mouth radiographic series on 242 women presenting for prenatal care. Fifteen percent (n=57) had an apical abscess and the suggested treatment was extraction of the affected tooth. Of those who were treated, none resulted in a miscarriage or stillbirth. Galloway summarized that removal of a known focal infection, which had clearly demonstrated to be a source of danger to any pregnant woman, was more beneficial than allowing the infection to harbor throughout the pregnancy. He went on to suggest that all foci of infection should be removed early in pregnancy.37
It is widely recognized that good oral health maintains the structures within the oral cavity. However, it is not universally accepted that oral health may be an independent contributor to abnormal pregnancy outcomes. Many studies have been conducted and the literature is controversial on the role periodontitis has and its influence on adverse pregnancy outcomes.
Myths regarding pregnancy and teeth:
- It is not true that you lose a tooth for every pregnancy. Decay is often the cause of tooth loss.
- Calcium is not taken from the mother's teeth for the baby's growth. This is provided through the mother's diet and if it is inadequate then it is taken from the mother's bone.
Recognition and understanding of the importance of oral health for systemic health has led to significant research into the role of maternal oral health and pregnancy outcomes. During pregnancy, changes in hormone levels promote an inflammatory response that increases the risk of developing gingivitis and periodontitis. As a result of varying hormone levels without any changes in the plaque levels, 50%-70% of all women will develop gingivitis during their pregnancy, commonly referred to as pregnancy gingivitis. This type of gingivitis is typically seen between the second and eighth month of pregnancy.38 Increased levels of the hormones progesterone and estrogen can have an effect on the small blood vessels of the gingiva, making it more permeable.39,40 This increases the mother's susceptibility to oral infections, allowing pathogenic bacteria to proliferate and contribute to inflammation in the gingiva. This hyperinflammatory state increases the sensitivity of the gingiva to the pathogenic bacteria found in dental biofilm. Females often see these changes during other periods of their life when hormones are fluctuating, such as puberty, menstruation, pregnancy, and again at menopause.39-41 Recent research suggests that the presence of maternal periodontitis has been associated with adverse pregnancy outcomes, such as preterm birth,19,20,23 preeclampsia,25 gestational diabetes,42 delivery of a small-for-gestational-age infant,14 and fetal loss.43 The strength of these associations ranges from a 2-fold to 7-fold increase in risk. The increased risks suggest that periodontitis may be an independent risk factor for adverse pregnancy outcomes.
In 1996, Offenbacher et al reported a potential association between maternal periodontal infection and delivery of a preterm or low-birth-weight infant.19 In a case-control study of 124 pregnant women, observations suggested that women who delivered at less than 37 weeks gestation or an infant weighed less than 2500 g had significantly worse periodontal infection than control women. In another case-control study conducted by Dasanayake, women who delivered a full-term infant weighing less than 2500 grams were matched to women who delivered full term infants weighing more than 2500 grams. All women received a periodontal evaluation after delivery, and poor periodontal health was determined to be an independent risk factor for delivering a low-birth-weight infant.22
Two prospective cohort studies23,44 found that moderate to severe periodontitis identified early in pregnancy is associated with an increased risk for spontaneous preterm birth, independent of other traditional risk factors. In the first study, investigators from the University of Alabama conducted a prospective evaluation of over 1300 pregnant women. Complete medical, behavioral, and periodontal data were collected between 21 and 24 weeks gestation. Generalized periodontal infection was defined as 90 or more tooth sites with periodontal ligament attachment loss of 3 mm or more. The risk for preterm birth was increased among women with generalized periodontal infection; this risk was inversely related to gestational age. After adjusting for maternal age, race, tobacco use, and parity, this relationship remained. The adjusted odds ratio for a preterm birth < 37 weeks for those women who now had generalized periodontal disease was 4.5 (95% CI, 2.2-9.2). The adjusted odds ratio increased to 5.3 (95% CI, 2.1-13.6) for preterm birth < 35 weeks gestation, and to 7.1 (95% CI, 1.7-27.4) for preterm birth < 32 weeks gestation.23
In the second study, Offenbacher et al44 conducted a prospective study of obstetric outcomes of over 1000 women who received an antepartum and postpartum periodontal examination. Moderate to severe periodontal infection was defined as 15 or more tooth sites with pockets depth greater than or equal to 4 mm. The incidence of increased periodontal pocketing, defined as clinical disease progression, was determined by comparing site-specific probing measurements between the antepartum and postpartum examinations. Disease progression was considered present if 4 or more tooth sites had an increase in pocket depths by 2 mm or more, with the postpartum probing depth being 4 mm or greater. Compared to women with periodontal health, the relative risk for spontaneous preterm birth < 37 weeks gestation was significantly elevated for women with moderate-severe periodontal infection (adj RR 2.0, 95% CI, 1.2-3.2), adjusting for maternal age, race, parity, previous preterm birth, tobacco use, markers of socioeconomic status, and presence of chorioamnionitis. Periodontal disease progression was found to be an independent risk factor for delivery < 32 weeks gestation (adj RR 2.4, 95% 1.1-5.2). The data from these 2 studies are important given the relationship between maternal periodontal disease and very preterm birth (< 32 weeks gestation), and the significant neonatal morbidity and mortality associated with very preterm birth.44
Santos-Pereira et al studied 124 women between the ages of 15-40 to determine if chronic periodontitis increased the risk of experiencing preterm labor (PTL). In this cross-sectional trial, women who were admitted for preterm labor, with intravenous tocolysis, were enrolled into the PTL group. The control group consisted of term pregnancies that were admitted following the PTL mother. Periodontal examinations were performed within 36-48 hours after delivery and before discharge. Chronic periodontitis was described as one site with clinical attachment loss (CAL) > 1 mm with gingival bleeding. The severity of periodontitis was classified as early (CAL < 3mm), moderate (CAL > 3 mm and < 5 mm), and severe (CAL > 5 mm). The extent of periodontitis was either localized, CAL < 30%, or generalized CAL > 30%. They concluded that chronic periodontitis increased the risk of having preterm labor {odds ratio of 4.7 (95% CI: 1.9-11.9)}, preterm birth {odds ratio 4.9 (95% CI: 1.9-12.8)}, and a low-birth-weight infant {OR 4.2(95% CI: 1.3-13.3)}.45
Pitiphat et al conducted a prospective study to determine if self-reported periodontitis was a risk factor for poor pregnancy outcomes. Women were enrolled prior to 22 weeks gestation and completed a self-report questionnaire during their second trimester. Demographic, medical and reproductive history, smoking, prepregnancy weight, and physical activity were assessed at the first prenatal visit. The self-reported questionnaire was validated by bitewing radiographs taken prior to delivery. The majority of the participants were white and middle class. Of the 354 participants who had bitewing radiographs available, the prevalence of self-reported periodontitis was 3.7%. It was noted that women who reported periodontitis had significantly higher mean radiographic bone loss than those that did not (p < 0.001). There was no significant increased risk of having a preterm birth or small-for-gestational-age infant when adjusting for smoking, race/ethnicity, socioeconomic status, BMI, history of preterm delivery, presence of genitourinary infection, weekly weight gain, and history of dental check-ups. However, there was a significant increase in risk for those who reported having periodontitis and poor pregnancy outcomes (adj OR 2.2: 95%CI 1.05-4.85). The authors concluded that periodontitis is an independent risk factor for poor pregnancy outcomes. However, caution should be taken when interpreting these results due to the sample size and the indirect measurement of periodontitis.46
In yet another prospective cohort, Agueda et al enrolled over 1200 women to evaluate the association between periodontitis and preterm birth and/or low birth weight. All women were between the ages of 18-40 and were enrolled between 20-24 weeks gestation. Demographic data, socioeconomic status, and medical and obstetric history were collected. Full mouth periodontal examinations, (PD, CAL, BOP) were performed by a single calibrated examiner and recorded at 6 sites per tooth. Periodontal disease was defined as 4 or more teeth with one or more sites with PD > 4 mm and CAL > 3 mm at the same site.14 After adjusting for confounding variables, a significant association was found between preterm birth and periodontitis (Adj OR 1.7 95% CI: 1.08-2.88). However no significant association was found between low birth weight and periodontitis.47
While there are data suggesting a relationship between maternal periodontal infection and preterm birth, several studies have failed to demonstrate such an association.31,42,48-50 In one of the largest studies to date, Moore et al examined the relationship between multiple periodontal parameters, including mean probing depths, percent of tooth sites with probing depths greater than or equal to 4 mm, percent of sites with bleeding on probing, and percent of sites with clinical attachment loss greater than or equal to either 2 or 3 mm. Moore found no difference in the periodontal parameters between women with preterm birth and without preterm birth.42 However, they did find a positive association between maternal periodontal infection and spontaneous abortion between 12 and 24 weeks (adj OR 2.5, 95% CI 1.2-5.4).43 In a case-control study, Budeneli and colleagues found no differences in periodontal infection between women who delivered preterm versus full term.49 However, women were at significantly increased risk for preterm birth if either P. gingivalis or C. rectus were found in the subgingival plaque.49
In a more recent case-control study, Vettore et al recruited 542 postpartum women who were over 30 years old.50 The investigators sought to explore the relationship between periodontal disease and preterm low birth weight. Cases were divided into 3 groups: low birth weight (n = 96), preterm (n = 110), and preterm and low birth weight (n = 63). Cases were compared to controls who were non-preterm and non-low-birth-weight individuals (n = 393). Periodontal measurements were collected and later stratified into 15 definitions of periodontal disease for analysis. Other covariates were also recorded and used for analysis. The results of this study indicated that periodontal disease levels were higher in control individuals than in cases, and that the extent of periodontal disease did not increase risk of preterm low birth weight. They also showed that in the preterm low birth weight group that the mean pocket depth and the frequency of sites with CAL > 3 mm were lower than in the control group. It was concluded that periodontal disease was not more severe in women with preterm low-birth-weight babies.51
Two recent meta-analyses of the association between maternal periodontal disease and preterm birth have been published. Vergnes et al examined 17 studies and reported a pooled estimate odds ratio for preterm birth of 2.83 (95% CI: 1.95-4.10, P < .0001).52 Xiong et al performed a systematic review and meta-analysis of 44 studies (26 case control, 13 cohort, and 5 controlled trials) to examine the relationship between maternal periodontal disease and adverse pregnancy outcome.53 The meta-analysis showed that maternal treatment of periodontal disease reduced the rate of preterm low birth weight infants as a group (pooled RR 0.53, 95% CI: 0.30-0.95, P < .05), but not preterm or low birth weight individually.
Inconsistencies with Previous Studies
While there are conflicting data regarding the association of periodontal diseases and adverse pregnancy outcomes, the reasons have yet to be identified. However, there are several differences and biases among the published data worth addressing. While the definitions of preterm birth, very preterm birth, low birth weight, small for gestational age, and other obstetric findings are well defined, no consensus has yet been achieved on the definition of periodontitis in periodontal research. A consensus on a definition is essential to optimize the interpretation, comparison, and validation of clinical data.54 With no universally agreed upon definition, any prior definitions may prove to be obsolete as we gain further information regarding the pathophysiology of the associations reported. Clinical markers of periodontal disease, such as gingival recession, clinical attachment loss, or bleeding on periodontal probing, may be late manifestations of the local infection, such that bacterial exposure may have already occurred with subsequent downstream deleterious effects. Recognition of the variation in clinical criteria used to define periodontal infection is important when critiquing the literature. In addition to the lack of a consistent clinical definition, several of the studies43,48,49 with no association between maternal periodontal disease and adverse pregnancy outcomes did not control for potential confounding variables. Another potential reason for the disparate findings among studies is the differences in populations studied. Most studies that showed an association between periodontal disease and adverse pregnancy outcomes have consistently been found in populations with a high incidence of preterm deliveries and within economically-challenged families. Quite the opposite is true for those studies that did not show an association. They were usually conducted in countries with universal health care and a lower incidence of preterm birth or low-birth-weight infants. Differential access to health care insurance, dental care, and prenatal care, may confound the relationship between maternal periodontal disease and adverse pregnancy outcome. Disparities in oral health may also be partially explained by racial differences in inflammatory and immune responses, as discussed previously (Table 1).
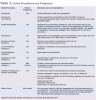
Another factor to consider when reviewing studies and synthesizing the results is the study design. The study design will influence the ability to reach a conclusion or determine causality. Case-control studies are limited in their experimental design because they cannot demonstrate causality. Prospective studies offer an advantage of studying the cause-effect relationship since the experiment can be designed and participants enrolled and followed over time with the outcome variable unknown at enrollment. Cohort studies involve 2 groups of people and compare a particular outcome of interest in groups that are alike in many ways but differ in some characteristics. Cross-sectional studies investigate a population at a point in time without regard to influencing factors that occurred prior to the study. The randomized clinical trial eliminates study bias by randomly assigning participants to the study groups. Neither the participant nor the researcher has any influence on which participant is assigned to each group. Random assignment to study groups prevents foreknowledge of study outcomes (Table 2).
Despite the controversy regarding the association between maternal periodontal infection and adverse pregnancy outcomes, several investigators have reported that periodontal treatment during pregnancy leads to a reduction in preterm birth risk.55-57 Lopez et al enrolled over 800 women in a randomized trial of periodontal treatment during pregnancy versus delayed treatment, and found almost a 5-fold reduction in preterm birth among women treated during pregnancy.55 In a pilot trial of periodontal treatment, Offenbacher et al found a trend toward reduced preterm birth among women treated during pregnancy compared with those who delayed therapy until postpartum This study demonstrated that women who were treated during pregnancy had a significant improvement in oral health measures and a reduction in oral pathogen burden.56 The women treated during pregnancy showed an improvement in clinical markers of periodontal infection, with reduction in clinical attachment loss and reduction in bleeding on dental probing. In another randomized, intent to treat study, Tarannum and Faizuddin found that nonsurgical periodontal treatment during pregnancy reduced the risk of preterm births (p < 0.001) and low birth weight (p < 0.002). An inverse correlation existed between CAL and birth weight in the control group, which may suggest that higher CAL were associated with lower birth weights. There was also an inverse correlation between gestational age and periodontal characteristic in both groups. This may suggest that shorter gestational ages were associated with higher values among periodontal parameters.58 These data are encouraging, as most periodontal diseases are both preventable and treatable, and thus would be of significant public health interest in pregnancy if a cause-effect relationship with preterm birth can be demonstrated.
However, excitement over periodontal treatment to prevent preterm birth must be tempered in light of a recently published study on periodontal treatment during pregnancy. Michalowicz et al studied 814 women at 3 clinical facilities.30 Women were randomized to scaling and root planing (SCRP) during before 21 weeks gestational age (treatment group) or after delivery (control group). Women in both groups, who experienced progressive periodontal disease defined as an increase of 3mm or more in clinical attachment loss, received SCRP in those areas. The study found no reduction in preterm births < 37 weeks gestation among women in the treatment group. On closer examination, there were almost twice as many deliveries that occurred before 32 weeks gestation among women in the control group (n=18) compared to women who were treated (n=10) during pregnancy. While not statistically significant, this is suggestive evidence that periodontal disease treatment might benefit those women at risk for the earliest and most morbid preterm births.
The data on the role of maternal periodontal infection and other adverse pregnancy outcomes are even less clear. Evidence suggests a role for inflammation and endothelial activation in the pathophysiology of preeclampsia;59,60 periodontal infection is one of many potential stimuli for these host responses. A 2-fold increased risk for preeclampsia was found among women with periodontal infection diagnosed at delivery.25 Others have also reported an association between maternal periodontal infection and preeclampsia.61,62 In a recent case-control study, Contreras et al62 found that women with preeclampsia were twice as likely to have chronic periodontitis. Also, preeclamptic women were more likely to have Porphyromonas gingivalis, Tannerella forsythensis, and Eikenella corrodens, known periodontal pathogens, compared to normotensive women. However, several other investigators have been unable to confirm an association between maternal periodontal infection and preeclampsia.63,64 The conflicting results have yet to be resolved. While other less common adverse pregnancy outcomes (eg, diabetes, small-for-gestational-age birth weight, miscarriage) may also be associated with maternal periodontal infection, data are currently too sparse to draw definitive conclusions regarding these associations and the potential benefits of treatment during pregnancy (Table 1).
Implications for Dental Hygiene Assessment, Diagnosis, and Treatment
Periodontal diseases are silent infections that have periods of exacerbation and quiescence that often go undiagnosed until irreparable damage occurs to the teeth and oral structures. Maintaining good oral hygiene before and during pregnancy is crucial for preventing gingivitis and periodontitis. Prevention and treatment of periodontal infection is aimed at controlling the bacterial biofilm, arresting progressive infection, and restoring lost tooth support.65 Dental professionals can facilitate this level of oral health through assessment, education, and proper treatment planning. Verifying the hormonal status and other risk factors for periodontal diseases and poor pregnancy outcomes of women during the medical history process will enable the provider to customize the treatment plan and oral hygiene instructions. Behavioral interventions such as smoking cessation, exercise, healthy diet, and maintenance of optimal weight are also useful preventive measures against periodontal disease.66 While the mechanisms of these interventions is unknown, they likely operate by reducing conditions that promote growth of pathologic bacteria, improving immune function, reducing inflammatory responses, and improving glucose control.
In 2004, the American Academy of Periodontology (AAP) issued a position statement regarding dental care for pregnant women. The AAP recommended that all women who were pregnant or planning a pregnancy should receive preventive dental care, including a periodontal examination, a prophylaxis, and restorative treatment. They also proposed that scaling and root planing should be completed early in the second trimester and that any presence of acute infection or abscess should be treated immediately, irrespective of gestational age. Treating infection as early as possible will remove a potential source of infection that could be harmful to the mother and the baby.67 In 2006, after a treatment trial30 failed to show an effect of scaling and root planing on birth outcomes, the AAP confirmed that treatment of periodontitis in pregnant women is safe and should be performed to improve the oral health of the woman.68 This conclusion was substantiated by Dr. Larry Tabak, director of the National Institutes of Dental and Craniofacial Research (NIDCR), when he said "Dental care during pregnancy has long been an issue dominated by caution more than data. The finding that periodontal treatment during pregnancy did not increase adverse events is important news for women, especially for those who will need to have their periodontal disease treated during pregnancy."69 The Academy of General Dentistry (AGD) recommends a dental visit for pregnant women or for those planning a pregnancy.70 Their recommendations are similar to the AAP but they suggest that pregnant women have a tiered treatment plan to include an examination in the first trimester, a dental cleaning in the second trimester, and then, depending on the patient, another appointment early in the third trimester.69 They also recommend communication between the dental provider and the obstetrician for any dental emergency that would require anesthesia or other medication to be prescribed. The American Dental Association (ADA) suggestions are similar to the AAP and the AGD; however, they also address the safety issues surrounding taking a dental radiograph during pregnancy. If a radiograph is needed for diagnosis or treatment, as they often are, then pregnant women should have the radiographs taken. Matteson et al estimated that a full mouth series of radiographs, with 20 radiographs, exposes the mother to < 1 mrem of radiation. The fetus is usually exposed to approximately 75 mrems of naturally occurring radiation during a pregnancy. Therefore, dental radiographs contribute to a negligible amount of radiation exposure.71 Care and caution should be taken to prevent further exposure by using a leaded apron with a thyroid collar.72
In 2004, Bright Futures Practice in Oral Health published an oral health pocket guide designed to provide health care providers with an overview of preventative oral health supervision for 5 developmental periods, including pregnancy and postpartum. Bright Futures began in 1990 and was initiated by the Health Resources and Services Administration (HSRA) Maternal and Child Health Bureau (MCHB). The guidelines suggest that health care providers assess the risk of oral disease and provide general suggestion to prevent carious lesions in pregnant women. Other suggestions or recommendations for the prevention of carious lesions included to expectorate and not rinse the mouth after brushing with a fluoridated toothpaste to allow the fluoride additional time to protect the teeth. They recommended that pregnant women use an alcohol-free, over the counter fluoridated mouth rinse at night. While carious lesions do not lead to periodontal diseases, the accumulation of bacterial plaque biofilm is a culprit in these diseases. Like many other initiatives, Bright Futures recommends that pregnant women visit an oral health care provider for an examination and restoration of all active carious lesions as soon as possible.73
First State Practice Guidelines for Treatment of Pregnant Patients
In 2006, the New York State Department of Health published practice guidelines for oral health care during pregnancy and early childhood. These guidelines were developed in response to a lack of information regarding the safety of dental treatment during pregnancy, which urged actions to reduce health disparities. These disparities were brought to national attention by the Surgeon General's Report, Oral Health in America,5 and a follow-up report titled "A National Call to Action to Promote Oral Health."74 The comprehensive guidelines provide by the New York Department of Health offers structure for oral health care providers so they can provide the best care for pregnant women. Providing dental care in pregnancy and early childhood are important to prevent lifelong consequences of poor oral health.73,75-79
Due to the reluctance of some dental professionals to provide dental care during pregnancy, the state of New York established guidelines to address this problem. This comprehensive report recommends that oral health care should be coordinated among prenatal and oral health care providers. Communication between the dental community and the medical community is a necessity and a consultation form was developed to help facilitate this process (Figure 1). The New York guidelines suggest and recommend that dental treatment be provided during pregnancy, including the first trimester. However, early in the second trimester (14-20 weeks gestation) is the most favorable time to perform dental procedures. During this gestational age there is no threat of teratogenicity, nausea and vomiting have usually subsided, and the uterus is below the umbilicus, providing more comfort to the mother. Unrestored carious lesions should be restored as soon as possible as some pregnant women require general anesthesia with intubation at delivery. Some physicians are hesitant to intubate due to the increased risk of airway obstruction due to the decreased integrity of decayed teeth that could break off. If treatment is provided in the last trimester, care should be taken to prevent suppression of the inferior vena cava by keeping the woman in an upright position. Ultimately all health care providers should advise women that maintaining good oral health during pregnancy is not only safe but necessary to reduce the risk of infection to the mother and possibly the fetus.
While it remains inconclusive whether maternal periodontal treatment improves pregnancy outcome, it is clear that treatment of varying degrees of clinical periodontal disease during pregnancy is safe and improves maternal oral health.56,57 In several studies of periodontal treatment during pregnancy, oral health parameters improved following therapy.30,56 All dental services should be available to pregnant women; however, studies have shown that some treatments are best provided only during certain gestational ages (Table 3). Despite the benefit of treatment, periodontal infection in women of childbearing age remains highly prevalent, particularly among low-income women and members of racial and ethnic minority groups. Regrettably, some subgroups of women who lack access to dental care will likely miss out on dental care during pregnancy. Oral health care professionals must help bridge this gap.
Dentists and dental hygienists must actively participate in providing treatment to pregnant women to help maintain maternal health. Knowledge of research studies (Table 1) and published guidelines can help eliminate the timidity that prevails in the dental community regarding providing dental care to pregnant women. In fact, the dental community must embrace this shift in practice guidelines. By embracing the changes, better overall health care can be provided to all women, especially those of child bearing age.
Oral Health Knowledge in the Medical Community
To provide better oral health care, more knowledge needs to be made available to the medical community. Few studies have tried to determine if the medical community has the knowledge to help educate patients about the importance of better oral care. Siriphant et al conducted focus groups with nurse practitioners (NP) in Maryland to determine the level of knowledge regarding oral cancer. They found nurse practitioners in Maryland did not recognize oral cancer as a health problem and that the main barrier for performing oral cancer screening was a lack of knowledge.80 In another survey of nurse practitioners, it was established that few recognized the signs of early oral cancer. NPs who reported attending a continuing education course on oral cancer within the last 2-5 years were 3.1 times more likely to have more knowledge regarding the risk factors for oral cancer and 2.9 times more likely to have more knowledge regarding risk factors and diagnostic procedures for oral cancer.81
Only a few studies have been reported in the literature that assess medical and nursing professionals' knowledge about periodontal disease and adverse pregnancy outcomes. Wilder et al surveyed practicing obstetricians in 5 counties in North Carolina to assess their knowledge of periodontal disease and to determine their practice behaviors regarding oral disease and adverse pregnancy outcomes. While 94 % of those surveyed could correctly identify bacteria as a cause of periodontitis, only 22% looked in a patient's mouth at an initial visit. And while most (84%) considered periodontal disease a risk factor for adverse pregnancy outcomes, 49% rarely or never recommended a dental visit during pregnancy.82 In a recent study conducted in North Carolina, 504 nurse practitioners, physician assistants and certified nurse midwives were surveyed. The survey assessed the knowledge, behavior, and opinions about periodontal disease and its relationship to adverse pregnancy outcomes. Forty eight percent responded (n = 204). Of those respondents, 63% reported looking in the patient's mouth to screen for oral problems at the initial visit. Twenty percent felt that their knowledge of periodontal disease was current, and all agreed that their discipline should receive instruction regarding periodontal disease. Ninety-five percent felt that a collaborative effort between the health care provider and the oral health care professionals was needed and would reduce the patient's risk of having an adverse pregnancy outcome.83 It is clear from the lack of studies available regarding oral health knowledge in the medical community that further studies are needed. One limitation to the future of oral health care is the lack of knowledge regarding oral care in the medical community. More education is needed within the medical community to help achieve better oral health care.
In a recent issue of the American Journal of Maternal Child Nursing, nurses were called to "action" to help facilitate better access to oral health care. Based on the surgeon general's report5 and the National Call to Action to Promote Oral Health,74 these authors suggested that nurses need to partner with other key stakeholders to prevent oral disease. The nurses were called to provide, promote, and protect women by increasing their knowledge, attitudes, awareness, and skills regarding oral health. By collaborating with other health professionals' access to oral health care can be improved.84
Providing oral health education in medical and nursing curricula might be one way to begin this process. A reported oral health curriculum at the University of Washington's medical school is reporting some success.85 In addition, the New York University Dental School is collaborating with the NYU School of Nursing to provide care to patients. This is a fundamental step in providing collaborative treatment to patients across many disciplines.84 Oral health care professionals can take the lead in educating other providers about the importance of oral health and what should be taught to pregnant women.
Future Projections in Care of Pregnant Patients
Amid the evidence that preventive and restorative dental services are beneficial for oral health and can help or modify systemic diseases, some insurance companies have begun to pay for expanded dental services.86 Insurance companies found that the cost of proving expanded dental services for some of its members decreases the amount spent on medical treatment.87 Based on this information, many companies have begun to offer additional dental benefits for those who have the most to gain such as pregnant women and patients with cardiovascular disease. While the literature is not clear on the association of periodontal disease and its effect on birth outcomes, it is clear the treating periodontal disease during pregnancy is beneficial for the mother and may be beneficial for the fetus. As part of these expanded services, Cigna, Delta Dental, United Health Care, and others have increased their dental benefits to include additional dental cleanings, including scaling and root planing as indicated for pregnant women. This represents a shift in the insurance industry that is beneficial to both the company and its members.87-89
Some state governments have answered the call to promote better oral health care by providing dental benefits to those who typically have none. In 2004, the Minnesota Department of Health partnered with the Minnesota Board of Dentistry and Minnesota Department of Human Services to make available resources and programs aimed at providing better access to dental care. This was accomplished by providing critical access dental provider designations, expanded authorization for dental hygienists and expanded duties for dental auxiliaries, a dental practice donation program, providing licensure of foreign trained dentists and retired dentists, and establishing a dentist loan-forgiveness program.90 In 2003, the Utah Department of Health (UDH) launched a program that served as a pilot study, which enabled pregnant women on Medicaid to receive dental examinations, treatment of decayed teeth, and a prophylaxis.91 UDH followed this up by expanding dental benefits available to Utah's pregnant Medicaid population. These women now have access to receive free dental check-ups, including x-rays, dental prophylaxis, restorations, root canals, and emergency treatment.91 As states and companies continue to expand their dental services provided for pregnant women, the overall health benefit will become apparent.
Future Directions for Research and Education
Future directions of oral health research should target oral health care before, during and after pregnancy. Studies that utilize the Centers for Disease Control's Pregnancy Risk Assessment Monitoring System (PRAMS) report that only 23%-43% of pregnant women receive dental care during pregnancy,92 a rate which is only one-half to two-thirds the overall use of dental services among US women.92 In addition, data explaining the racial/ethnic disparities in oral health among pregnant women are lacking. Pregnant women's perceptions of oral health, and the barriers and motivations to their seeking dental care, must be assessed to adequately introduce preventive information on oral health into their prenatal care, which is one of the first steps in reducing health disparities.
Further studies are needed to better understand the mechanism of periodontal disease-associated preterm birth and to tailor treatment to those women who might benefit the most. Confirmation of periodontal infection as an independent risk factor for adverse pregnancy outcomes and identification of those at greatest risk would be of significant public health importance because periodontal infection is both preventable and curable. At present, however, there is insufficient evidence for health care policy recommendations to provide maternal periodontal treatments for the purpose of reducing the risk of adverse pregnancy outcome regardless of its other benefits.
Further educational opportunities need to be provided for allied health professionals and the medical community to help alleviate the problems with access to dental care. Relationships between professional schools need to be forged so that cross-educational opportunities can be provided to all disciplines. Training and education should be expanded to prepare dental hygienists to partner with physicians and nurse practitioners to provide a minimum level of care for those who have no access to dental care. These services could include an oral screening, oral hygiene instructions, toothbrush prophylaxis, referrals if needed, application of fluoride, and nutritional counseling. The dental community could partner with the medical community to provide dental and medical services within the same office, providing better access to care.
Given the relationship between maternal and infant oral health and periodontal infection and general health and well-being, oral health care should be a goal in its own right for all individuals, including reproductive-aged and pregnant women. There is no evidence to suggest that dental examination or treatment is deleterious to the pregnant woman or her developing fetus. Infective endocarditis prophylaxis is recommended for all dental procedures for those individuals at high risk for infective endocarditis. Pregnant women who meet American Heart Association guidelines for infective endocarditis prophylaxis93 and undergo these dental procedures should be treated similar to nonpregnant individuals.
Regardless of the potential for improved oral health to improve pregnancy outcomes, public policies that support comprehensive dental services for vulnerable women of childbearing age should be expanded so that their own oral and general health is safeguarded, and the morbidity of childhood caries reduced. Mechanisms to educate women and their health care providers about the importance of oral health need to be in place, and improvement in the access to care for all must occur if oral health interventions are to make an important impact on pregnancy outcomes.
Conclusion
The importance of providing oral health care for pregnant women cannot be disputed. Data suggest that maternal oral health impacts pregnancy health; further research on the causal nature of this association is ongoing to determine if there is a relationship. Current guidelines and data suggest that dental care during pregnancy is safe. However, scaling and root planing is best accomplished between 14-20 weeks gestational age. Providing dental care for pregnant women will help remove potentially harmful bacteria from dissemination and possibly leading to other complications. As oral health care providers we can educate our patients regarding the importance of oral health and on important preventive measures to maintain oral health
References
1. Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006;137(supp):10S-15S.
2. Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the atherosclerosis risk in communities study. Arch Intern Med. 2003; 163(10):1172-9.
3. Periodontal Diseases. Chicago, Ill. American Academy of Periodontology. www.perio.org/consumer/2a.html.
4. Periodontal (Gum) Disease: Causes, Symptoms, and Treatments. Bethesda, Md. National Institute of Dental and Craniofacial Research. www.nidcr.nih.gov/.
5. Oral health in America: A report of the Surgeon General. Rockville, Md. US Department of Health and Human Services. www.surgeongeneral.gov/library/oralhealth/
6. Philstrom B, Michalowixz BS, Johnson NW. Periodontal Diseases. Lancet. 2005;366(9499):1809-20.
7. Albandar JM, Brunelle JA, Kingman A. Destructive Periodontal Disease in Adults 30 Years of Age and Older in the United States, 1988-1994. J Periodontol. 1999;70(1):13-9.
8. Spahr A, Klein E, Khuseyinova N, et al. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch Intern Med. 2006;166(5):554-9.
9. Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol. 2006; 77(7):1173-8.
10. Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol. 2006;33(6):408-14.
11. Al-Shammari KF, Al-Ansari JM, Moussa NM, Ben-Nakhi A, Al-Arouj M, Wang HL. Association of periodontal disease severity with diabetes duration and diabetic complications in patients with type 1 diabetes mellitus. J Int Acad Periodontol. 2006;8(4):109-14.
12. Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465-82.
13. Beck JD, Eke PI, Heiss G, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005(1);112:19-24.
14. Boggess KA, Beck JD, Murtha AP, et al. Maternal periodontal disease in early pregnancy and risk for a small-for-gestational-age infant. Am J Obstet Gynecol. 2006;194(5):1316-22.
15. Lopez NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res. 2002;81(1):58-63.
16. Dasanayake AP, Russell S, Boyd D, et al. Preterm low birth weight and periodontal disease among African Americans. Dent Clin North Am. 2003;47(1):115-25, x-xi.
17. Goepfert AR, Jeffcoat MK, Andrews WW, et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol. 2004;104(4):777-83.
18. Kunnen A, Blaauw J, van Doormaal JJ, et al. Women with a recent history of early-onset pre-eclampsia have a worse periodontal condition. J Clin Periodontol. 2007;34(3):202-7.
19. Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67(10 suppl):1103-13.
20. Offenbacher S, Lieff S, Boggess KA, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6(1):164-74.
21. Madianos PN, Lieff S, Murtha AP, et al. Maternal periodontitis and prematurity: Part II. Maternal infection and fetal exposure. Ann Periodontol. 2001;6(1):175-82.
22. Dasanayake AP. Poor periodontal health of the pregnant woman as a risk factor for low birth weight. Ann Periodontol. 1998;3(1):206-12.
23. Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg R and Hauth JC. Periodontal infection and preterm birth:Results of a prospective study. J Am Dent Assoc. 2001;132(7);875-880.
24. Romero BC, Chiquito CS, Elejalde LE, Bernardoni CB. Relationship between periodontal disease in pregnant women and the nutritional condition of their newborns. J Periodontol. 2002;73(10):1177-83.
25. Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101(2):227-31.
26. D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006;15:(5):977-84.
27. Faria-Almeida R, Navarro A, Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis. J Periodontol. 2006;77(4):591-8.
28. Genuit T, Bochicchio G, Napolitano LM, McCarter RJ, Roghman MC. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg Infect (Larchmt). 2001;2(1):5-18.
29. Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med.2006;173(12):1348-55.
30. Michalowicz BS, Hodges JS, Di Angelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355(18):1885-94.
31. Davenport ES, Williams CE, Sterne JA, Murad S, Sivapathasundram V, Curtis MA. Maternal periodontal disease and preterm low birthweight: case-control study. J Dent Res 2002;81(5):313-8.
32. Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol. 2005;76(11 suppl):2144-53.
33. Laine MA. Effect of pregnancy on periodontal and dental health. Acta Odontol Scand. 2002;60(5):257-64.
34. Final Natality Data. Hyattsville, Md. National Center for Health Statistics. www.cdc.gov/nchs/births.htm
35. Andrews WW, Hauth JC, Goldenberg RL. Infection and Preterm Birth. Amer J Perinatol. 2000;17(7):357-65.
36. Raju TN. Late-preterm births: challenges and opportunities. Pediatrics. 2008;121(2);402-3.
37. Galloway CE. Focal Infection. Am J Surg. 1931;14(3):643-645.
38. Pregnancy and Swollen Gums. Irving, Tex. American Pregnancy Association. www.americanpregnancy.org/pregnancyhealth/swollengums.html.
39. Jensen J, Liljemark W, Bloomquist C. The effect of female sex hormones on subgingival plaque. J Periodontol. 1981;52(10):599-601.
40. Barak S, Oettinger-Barak O, Oettinger M, Machtei EE, Peled M, Ohel G. Common oral manifestations during pregnancy: a review. Obstet Gynecol Surv. 2003;58(9):624-8.
41. Protecting oral health throughout your life. Chicago, Ill. American Academy of Periodontology. www.perio.org/consumer/women.htm
42. Xiong X, Buekens P, Vastardis S, Pridjian G. Periodontal disease and gestational diabetes mellitus. Am J Obstet Gynecol. 2006;195(4):1086-9.
43. Moore S, Ide M, Coward PY, et al. A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. Br Dent J. 2004;197(10):251-8; discussion 247.
44. Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107(1):29-36.
45. Santos-Pereira SA, Giraldo PC, Saba-Chujfi E et al: Chronic periodontitis and pre-term labour in Brazilian pregnant women: an association to be analysed. J Clin Periodontol. 2007;34(3):208-13.
46. Pitiphat W, Joshipura KJ, Gillman MW, et al. Maternal periodontitis and adverse pregnancy outcomes, Community Dent Oral Epidemiol. 2008;36(1):3-11.
47. Agueda A, Ramon JM, Manau C, Guerrero A, Echeverria JJ. Periodontal disease as a risk factor for adverse pregnancy outcomes: a prospective cohort study. J Clin Periodontol. 2008;35(1):16-22.
48. Holbrook WP, Oskarsdottir A, Fridjonsson T, Einarsson H, Hauksson A, Geirsson RT. No link between low-grade periodontal disease and preterm birth: a pilot study in a healthy Caucasian population. Acta Odontol Scand. 2004;62(3):177-9.
49. Buduneli N, Baylas H, Buduneli E, Turkoglu O, Kose T, Dahlen G. Periodontal infections and pre-term low birth weight: a case-control study. J Clin Periodontol. 2005;32(2):174-81.
50. Rajapakse PS, Nagarathne M, Chandrasekra KB, Dasanayake AP. Periodontal disease and prematurity among non-smoking Sri Lankan women. J Dent Res. 2005;84(3):274-7.
51. Vettore MV, Leal M, Leão AT, et al. The relationship between periodontitis and low birth weight. J Dent Res. 2008;87(1):73-8.
52. Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol. 2007;196(2):135. e1-7.
53. Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113(2):135-43.
54. Borrell L, Papapanou PN. Analytical epidemiology of periodontitis. J Clinic Periodontol. 2005:32(6 suppl)132-158.
55. Lopez NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol. 2002;73(8):911-24.
56. Offenbacher S, Lin D, Strauss R, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. J Periodontol. 2006;77(12):2011-24.
57. Jeffcoat MK, Hauth JC, Geurs NC, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74(8):1214-8.
58. Tarannum F, Faizudin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J Periodontol. 2007; 78(11):2095-2103.
59. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200-4.
60. Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. 2005;84(8):788-93.
61. Canakci V, Canakci CF, Canakci H, et al. Periodontal disease as a risk factor for pre-eclampsia: a case control study. Aust N Z J Obstet Gynaecol. 2004;44(6):568-73.
62. Contreras A, Herrera JA, Soto JE, Arce RM, Jaramillo A, Botero JE. Periodontitis is associated with preeclampsia in pregnant women. J Periodontol. 2006;77(2):182-8.
63. Khader YS, Jibreal M, Al-Omiri M, Amarin Z. Lack of association between periodontal parameters and preeclampsia. J Periodontol. 2006;77(10):1681-7.
64. Meurman JH, Furuholm J, Kaaja R, Rintamaki H, Tikkanen U. Oral health in women with pregnancy and delivery complications. Clin Oral Investig. 2006;10(2):96-101.
65. Jeffcoat MK. Prevention of periodontal diseases in adults: strategies for the future. Prev Med. 1994;23(5):704-8.
66. Al-Zahrani MS, Borawski EA, Bissada NF. Periodontitis and three health-enhancing behaviors: maintaining normal weight, engaging in recommended level of exercise, and consuming a high-quality diet. J Periodontol. 2005;76(8):1362-6.
67. American Academy of Periodontology statement regarding periodontal management of the pregnant patient. J Periodontol. 2004;75(3):495.
68. American Academy of Periodontology Statement on Periodontal Disease and Preterm Low Birthweight. Chicago, Ill. American Academy of Periodotnology. www.perio.org/consumer/nejm-statement.htm.
69. Study Finds Periodontal Treatment Does Not Lower Preterm Birth Risk. Bethesda, Md. National Institute of Dental and Craniofacial Research. www.nih.gov/news/pr/nov2006/nidcr-01.htm
70. How does pregnancy affect my oral health? Chicago, Ill. Academy of General Dentistry. www.agd.org/public/oralhealth/Default.asp?IssID=341&Topic=W&ArtID=1372
71. Matteson SR, Joseph LP, Bottomley W, et al. The report of the panel to develop radiographic selection criteria for dental patients. Gen Dent. 1991;39(4):264-70.
72. The selection of patients for dental radiographic examinations. Chicago, Ill. American Dental Association. www.ada.org/sections/professionalResources/pdfs/topics_radiography_examinations.pdf.
73. Casamassimo P. Bright Futures in Practice: Oral Health. Arlington, Va. National Center for Education in Maternal and Child Health. 1996.
74. A National Call to Action to Promote Oral Health. Rockville, Md. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health, National Institute of Dental and Craniofacial Research. May 2003.
75. Casamassimo P. Oral Health and Learning. Bright Futures in Practice: Oral Health. Arlington, Va. National Center for Education in Maternal and Child Health. 1996.
76. Lewit EM, Monheit AC. Expenditures on Health Care for Children and Pregnant Women. Future Child 1992;2(2):95-114.
77. The Face of a Child: Surgeon General's Workshop and Conference on Children and Oral Health, Proceedings. Bethesda, Md. National Institute of Dental and Craniofacial Research. June 2000. www.nidcr.nih.gov/NR/rdonlyres/ED6FB3B5-CEF4-4175-938D-5049D8A74F66/0/ SGR_Conf_Proc.pdf.
78. Gajendra S, Kumar JV. Oral health and pregnancy: a review. N Y State Dent J. 2004;70(1):40-44.
79. Edelstein BL. Foreword to the Supplement on Children and Oral Health. Ambul Pediatr. 2002;2(2 suppl):139-140.
80. Siriphant P, Horowitz AM, Child WL. Perspectives of Maryland adult and family practice nurse practitioners on oral cancer. J Public Health Dent. 2001;61(3):145-9.
81. Siriphant P, Drury TF, Horowitz AM, Harris RM. Oral cancer knowledge and opinions among Maryland nurse practitioners. J Public Health Dent. 2001;61(3):138-44.
82. Wilder R, Robinson C, Jared HL, Lieff S, Boggess K. Obstetricians' knowledge and practice behaviors concerning periodontal health and preterm delivery and low birth weight. J Dent Hyg. 2007; 81(4):81. Epub 2007 Oct 1.
83. Thomas KM, Jared HL, Boggess K, Lee J, Moos M, Wilder RS. Prenatal Care Providers' Oral Health and Pregnancy Knowledge Behaviors. J Dent Res. 2008;87(Spec Iss A).
84. The American Journal of Maternal Child Nursing. Jan/Feb 2008;33(1)6-64.
85. Mouradian WE, Reeves A, Kim S, et al. A new oral health elective for medical students at the University of Washington. Teach Learn Med. 2006;18(4):336-42.
86. Several Large Health Insurers Expand Dental Coverage for Members. Kaiser Daily Health Policy Report. September 19, 2006. www.kaiserhealthnews.org/daily-reports/2006/september/19/dr00039904.aspx?referrer=search
87. Lieberman S. Cigna weighs in on oral-systemic medicine. Grand Rounds in Oral-Systemic Medicine. July 2007. www.dentistryiq.com/index/display/article-display/298128/articles/grand-rounds-in-oral-systemic-medicine/volume-2/issue-2/guest-editorial/cigna-weighs-in-on-oral-systemic-medicine.html
88. Delta Dental Insurance and Delta Dental of Pennsylvania add additional benefits for expectant mothers and implant coverage. October 2007. Business Wire.
89. United Healthcare Dental Prenatal Dental Care Program. Minneapolis, Minn. April 2007. www.unitedhealthcarenortheast.com/Seminars/Fall07/Collateral/UnitedHealthcare.Prenatal%20Dental.One.Sheet.pdf
90. Pregnant women, mothers and infants: dental health for women. St. Paul, Minn. Minnesota Department of Health.
91. UDOH recommends second trimester dental cleanings to help reduce the chance of babies born too early and too small. Salt Lake City, Utah. Utah Department of Health.
92. Gaffield ML, Gilbert BJ, Malvitz DM, Romaguera R. Oral health during pregnancy: an analysis of information collected by the pregnancy risk assessment monitoring system. J Am Dent Assoc 2001;132(17):1009-16
93. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. A Guideline From the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2008;139(suppl):3S-24S.
Additional Resources
Web sites
American Dental Hygienists' Association www.adha.org
National Institutes of Health www.nih.gov
National Institute of Dental and Craniofacial Research www.nidcr.nih.gov
Centers for Disease Control and Prevention www.cdc.gov
American Dental Association www.ada.org
American Academy of Periodontology www.perio.org
NY State Oral Health Care during Pregnancy and Early Childhood Practice Guidelines www.health.state.ny.us/publications/0824/pda/windows_mobile/0824.pdf
Oral Health in America: A Report of the Surgeon General (executive summary) www.nidrc.hig.gov/AboutNIDRR/Surgeon General/ExecutiveSummary.htm
American Pregnancy Association www.americanpregnancy.org
Academy of General Dentistry www.agd.org
Healthy People 2010: Section 21, Oral Health www.healthypeople.gov/Document/HTML/Volume2/21Oral.htm
Oral Health America www.oralhealthamerica.org
Maternal and Child Health Library: Knowledge Path – Oral Health and Children and Adolescents www.mchlibrary.info/KnowledgePaths/kp_oralhealth.html
Children's Dental Health Project www.cdhp.org
Brochures
Dental Care for Your Baby American Academy of Pediatric Dentistry www.aapd.org/publications/brochures/babycare.asp
A Healthy Mouth for Your Baby National Institutes of Health www.nidcr.nih.gov/HealthInformation/DiseasesAndConditions/ChildrensOralHealth/Healthy- Mouth/default.htm
Definitions: Terms Used in Periodontitis and Pregnancy Outcomes Studies
Antepartum: Time between conception and the onset of labor; usually used to describe the period when a woman is pregnant.
Chorioamnionitis Inflammation of the chorion and the amnion, the membranes that surround the fetus. Chorioamnionitis usually is associated with a bacterial infection. This may be due to bacteria ascending from the mother's genital tract into the uterus to infect the membranes and the amniotic fluid. Chorioamnionitis is dangerous to the mother and child. It greatly increases the risk of preterm labor and, if the child survives, the risk of cerebral palsy.
HbA1c levels HbA1c is a test that measures the amount of glycosylated hemoglobin in the blood. Glycosylated hemoglobin is a molecule in red blood cells that has glucose (blood sugar) attached to it. A person will have more glycosylated hemoglobin if they have more glucose in their blood for long periods of time. The test gives a good estimate of how well diabetes has been managed over the previous 2 or 3 months.
Inflammatory cytokines Proteins produced predominantly by activated immune cells that are involved in the amplification of inflammatory reactions. Low birth weight Any birth when the infant weighs less than 2500 grams (5 pounds 8 ounces)
Normotensive Normal blood pressure
Post partum In the period after delivery
Preeclampsia A condition in pregnancy characterized by abrupt hypertension (a sharp rise in blood pressure), albuminuria (leakage of large amounts of the protein albumin into the urine) and edema (swelling) of the hands, feet, and face. Preeclampsia is one of the most common complications of pregnancy. It affects about 5% of pregnancies. It usually occurs in the third trimester of pregnancy.
Pregnancy gingivitis Gingivitis in which the host response to bacterial plaque is presumably exacerbated by hormonal alterations occurring during puberty, pregnancy, oral contraceptive use, or menopause.
Preterm birth Any birth prior to 37 weeks gestational age
Teratogenicity The capability of producing fetal malformations
Very preterm birth Any delivery of a live born infant less than 32 weeks gestational age
About the Authors
Heather Jared, BSDH, MS, is a research associate professor in the Department of Dental Ecology and conducts research in the Center for Oral Systemic Disease at the University of North Carolina School of Dentistry, Chapel Hill, NC.
Kim A. Boggess, MD, is an associate professor of Obstetrics & Gynecology in the Division of Maternal-Fetal Medicine at the University of North Carolina in Chapel Hill, NC.