You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
The negative effects of ionizing radiation on human tissues of both patients and operators have been well documented.1,2 As a result, dentistry has tried to minimize patient dose through the use of patient protective equipment, faster receptors, digital imaging, beam alignment devices, longer source to end distances, and collimation of the beam.1-5 Several decades ago, beam alignment paralleling instruments were introduced on the commercial market to minimize patient dose and improve diagnostic quality. Originally, these were used with circular collimation. In the 1980s, a universal rectangular collimator was developed for use with beam alignment devices to further reduce the dose to the patient. Although successful in reducing dose, studies have shown that rectangular collimation has not been well adopted by the majority of dental practitioners and its use results in more collimator cut errors. The most likely explanation for this occurrence is the reduced margin for error.5-7
The American Dental Association (ADA), International Commission on Radiation Protection (ICRP), and National Commission on Radiation Protection (NCRP) strongly recommend the use of rectangular collimation with intraoral imaging.1-4 A current guideline established by the NCRP states that the x-ray beam should not exceed the minimum coverage necessary, and each dimension of the beam should be collimated so that the beam does not exceed the receptor by more than 2% of the source-to-image receptor distance. Radiographic equipment is either manufactured to incorporate rectangular collimation or universal adapters are available to retrofit existing circularly collimated equipment.5,6 Continuing concern about long-term and cumulative risks of cancer development from low doses of ionizing radiation has increased interest in the implementation of rectangular collimation.1
More recently, a rectangular collimator device has been marketed with enhancement features that may help minimize the collimator cut technique errors created with a rectangular collimated beam. A device composed of a magnetic alignment ring and a positioning-indicator laser beam with a visual light and audible signal was designed to eliminate collimator cuts and retakes. An early study evaluated technical performance using the device prototype and the authors recommended modifications to optimize the diagnostic quality of the image.5 These modifications allowed for retrofitting the device to circular collimators and an increased size of the rectangular window. This study’s authors are unaware of any studies that have evaluated how these design changes affected the ability to produce quality and diagnostic intraoral images with this device. Therefore, the purpose of this study was to evaluate the technical performance of two rectangular collimator modalities currently available on the commercial market.
Methods and Materials
The study population consisted of 33 senior dental hygiene students at the University of North Carolina at Chapel Hill School of Dentistry. Criteria for inclusion in the study were successful completion of the preclinical radiology course and two semesters of clinical radiology experience prior to enrolling to participate. Participants were asked to enroll voluntarily in the study and sign a consent form. This study was approved by the University of North Carolina Institutional Review Board.
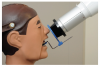
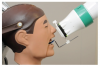
Two device/collimator combinations were used to test for technical performance and diagnostic acceptability. Both device combinations were designed to be used with the Rinn XCP® receptor holding device, although the method for alignment varied depending on the device employed. The Rinn® universal rectangular collimator insert (Rinn Corp, Elgin, Illinois) hereafter referred to as “Universal” was fitted over the circular collimator end resulting in a 33-cm source-to-end distance (Figure 1a). The universal collimator produced an exposure area of 46 mm x 36 mm (1,652 mm2), measured at a distance of 2.5 cm from the collimator end.8 The IDI Tru-Align™ intraoral rectangular collimating device, hereafter referred to as “Enhanced,” was fitted on the opening of the tube head producing a 30-cm source-to-end distance (Figure 1b). The enhanced device produced an exposure area of 56 mm x 45 mm (2,524 mm2), measured at a distance of 2.5 cm from the collimator end.8 The universal device was used with the XCP® receptor holding device (receptor holder/bite block with corresponding alignment ring and bar). For techniques used with the enhanced device, the XCP® ring was replaced by a specifically designed ring (Figure 2). The enhanced device’s alignment ring was square in shape with the appropriately corresponding appendages for anterior/bitewing and posterior projections. The alignment ring was affixed with multiple round flush mounted magnets to secure the collimator to the aiming device (Figure 3).
All projections were exposed using DenOptix® (GENDEX, Hatfield, Pennsylvania) Photostimulable Phosphor Plate (PSP) receptors for each FMX. Size 1 receptors were used for lateral/canine periapical projections (n = 4) and Size 2 receptors were used for central (n = 2), premolar (n = 4), and molar (n = 4) periapical projections and premolar (n = 2) and molar (n = 2) bitewing projections. A total of 18 projections constituted an FMX for the technical performance segment of this study.
All exposures with both universal and enhanced collimator devices were made using an intraoral Planmeca Prostyle x-ray unit (Intra, Planmeca USA, Roselle, Illinois). Two Dental X-ray Teaching and Training Replicas (DXTTRs) (RinnCorp, Elgin, Illinois) were identified for use in the study. Each DXTTR was designed with natural teeth and human skulls. Selection of the DXTTRs was based on optimal, mechanical, and operational conditions.
A five-item post-participation survey instrument was designed to solicit information from the subjects regarding their experience using the universal and enhanced devices. All five questions were open-ended in design. One asked for any complications or malfunctions that may have occurred with either device. The second asked for the helpfulness of the added features of the enhanced device (visual, audible, magnetic ring). Two of the questions explored the subject’s impression of the image quality rendered and ease of use of both collimators. The last question asked the operator overall preference for their choice of device and why.
All study subjects chose a block of time to participate. No more than two subjects could participate at the same time. Once a time for participation was established, each subject was required to consent by reading and signing the IRB approved study participation consent form. Upon arrival, subjects were given a brief review on the proper usage of each of the two devices and their task. Prior to arrival, the principal investigator set up DXTTR manikins, arranged sensors with a corresponding FMX template, and installed both universal and enhanced devices to be ready for use. Each subject was randomly assigned to an operatory, DXXTR manikin, and one of two study devices. When ready to begin, consented subjects exposed one FMX using either the universal device or the enhanced device. The principal investigator recorded start and stop times for each study subject during testing of each device. Upon completion of the first FMX with either device, the principal investigator gathered exposed sensors and scanned images into the Training Electronic Patient Record (TEPR). All images were coded to blind the evaluator to the subject and device used. The principal investigator removed the first of the two devices tested and installed the remaining device for subject use and start and stop times were again recorded. Subjects were allowed unlimited time to complete the 18 projection series, but were encouraged to treat the radiographic exam as if it were a patient simulation. Both FMX’s were exposed using PSP digital sensors on a DXTTR manikin. At the end of their task, each subject completed the post participation survey. Each survey document was coded providing anonymity for the study subject while offering the principal investigator identification of device, DXTTR and operatory used.
An experienced evaluator (dental hygiene professor with 35 years’ experience evaluating radiographic technical performance) assessed the radiographic images for technical and diagnostic quality. Intra-rater reliability was determined during the evaluation process by randomly re-grading 10 FMX’s (five with the universal collimator and five with the enhanced collimator). Each projection was viewed in a low-lit room on a 22” Lenovo monitor with a resolution of 1680 x 1050 dpi. All projections were evaluated over a 3-hour time frame with periodic (two 10-minute) breaks. Data were collected using a direct data entry system using an EXCEL (Microsoft 2010 Version) statistical application.
All study images were blinded to the evaluator based on device/collimator combination and radiographer. The images were evaluated based on predetermined criteria assessing the presence and severity of collimator centering (CC), vertical angulation (V), horizontal angulation (H), and packet placement (PP) errors. If the error was present but the projection was diagnostically acceptable, then the error was coded as a “minor” error. If the error was present but rendered the projection diagnostically unacceptable, then the error was coded a “major” error. Minor errors involving packet placement, horizontal angulation, vertical angulation, and collimator centering constituted a deduction of one point per error with 4 points being the greatest deduction per projection. Major errors involving any of the 4 criteria were deemed non-diagnostic and automatically resulted in a 4-point deduction for that image. Each of the 18 images of the FMX was graded and an overall score given for that set of images.
Data were analyzed using frequencies, ANOVA, and least squares means using a general linear model. The mean number of errors per full-mouth series were calculated and then averaged across all full-mouth series. A general linear model was used to analyze mean numbers of errors between the two devices. ANOVA was used to assess error differences due to location in the mouth (Anterior, Posterior, and Bitewing). A paired t-test was used to evaluate the mean time/effort between the two devices. Intra-rater reliability was measured using an Intraclass Correlation Coefficient (ICC).
Results
A total of 17 subjects enrolled in the study comprising a 51.5% participation rate. All subjects completed the technical component of the study and the post-participation survey. The intra-rater reliability was ICC = 0.77.
Figure 4 and Figure 5 present the findings of all errors by number and error type. Figure 4 displays the mean number of errors per full mouth for all technique errors (PP, V, H, and CC) as a function of the collimator device (universal vs. enhanced). The mean number of errors per FMX for the universal device was 12.1 and 9.7 for the enhanced device. A statistically significant difference was seen when the data were analyzed using the adjusted model (F = 4.35, df = 1, P = .048). In Figure 5, the data were evaluated by the mean number of errors per full mouth as a function of error type by device, a similar number of errors occurred by device for each error type except for collimator centering. The mean number of collimator centering errors per full-mouth series occurred three times more often with the universal device (universal device = 3.6 vs. enhanced device = 1.1).
Figure 6 presents the findings based on error severity (major or minor) displaying the average number of errors (PP, V, H, and CC) per FMX. An error scored as a major error indicated that the image did not offer diagnostic value. A minor error indicated that the error was present but did not compromise the diagnostic quality of the image. The mean number of diagnostically unacceptable errors per full-mouth series was similar between the devices (universal device = 3.2 vs. enhanced device = 2.9). A greater difference was seen in the reported mean number of minor errors per full mouth between the two devices (universal device = 8.9 vs. enhanced device = 6.8). Minor collimator centering errors occurred three times more often with the universal device (universal device = 3.5 vs. enhanced device = 1.1).
Figure 7 shows the error rates based on location. The average number of all errors that occurred was evaluated based on location in the mouth (Anterior, Posterior, Bitewing) and by device (universal vs. enhanced). There was a difference in the average number of errors when comparing posterior (universal device = 6.5 vs. enhanced device = 5.4) to anterior locations (universal device = 2.5 vs. enhanced device = 2.0) and posterior to bitewing locations (universal device = 3.1 vs. enhanced device = 2.3). The model showed a statistically significant difference in the average number of errors per FMX when comparing posterior to anterior locations and posterior to bitewing locations (P < .0001). There was not a significant difference when comparing anterior to bitewing locations (P > .38).
Time required to complete a FMX by device was evaluated. Average time required to complete a FMX using the universal and enhanced device was 21 minutes and 17 minutes respectively. Significantly less time was needed (4 minutes) to expose a FMX when using the enhanced device (P = .0001) (Figure 8).
Table I displays the subject responses to each of the five questions of the post-participation survey. Question #1 asked the subjects (n = 17) to state any complications/malfunctions of the device/collimator combinations that were experienced when exposing the projections. Regarding the universal device, four subjects (24%) reported x-ray unit tube head instability or drifting and one subject (<1%) reported experiencing a malfunction with the collimator. Regarding the enhanced device, 8 subjects (47%) reported that the weight of the device was an issue and 6 six subjects (35%) reported that the lighted signal feature produced inaccuracies.
Question #2 asked the subjects (n = 17) to list which enhancement features (audible and visual signals, magnetic ring), if any, were helpful to them as the operator. Eighty-two percent chose the visual (lighted) signal, 71% listed the magnetic positioning ring, and 35% listed the audible signal as being helpful to them during exposures.
Questions 3 and 4 explored the choices of subjects regarding impact on image quality and ease of use. Responses to Question 3 indicated that fifteen subjects felt that using the enhanced device would produce better quality images. One subject chose the universal device and one subject remained undecided. Question 4 asked the subjects (n = 17) to make a choice as to which of the two devices they found easier to use. Sixteen chose the enhanced device while 1 one remained undecided. No subjects chose the universal device.
Question 5 asked the subjects (n = 17) to choose a device based on their overall preference and to elaborate as to why. Sixteen responses were in favor of the enhanced device while one subject preferred the universal device. Explanations that subjects provided for preference of the enhanced device were that it provided confidence to the operator regarding exposure of a quality image, less time, and ease of use. The one subject that preferred the universal device made this decision based on familiarity with the device.
Discussion
A primary goal of radiography is to render a diagnostic image while keeping the dose to the patient as low as reasonably achievable. This study compared the technical outcome of two rectangular collimators: one with technique enhancement features that retrofitted to the tubehead and one that inserted into a circular collimator. In addition, subject feedback was solicited on the use and preference of the collimators.
When the devices were compared based on technical performance there was not a consistent pattern seen where one device outperformed the other with respect to packet placement, vertical angulation, or horizontal angulation errors. However, the enhanced device produced significantly fewer overall errors when compared to the universal device. The type of error that was primarily reduced with the enhanced device was collimator cutting. This finding is in contrast to that reported by Zhang et al.5 Zhang’s study reported an increase in collimator cuts and suggested that the device may be modified to increase the aperture opening in the device.5 Interestingly, the current study discovered that there was minimal difference between the devices in the number of errors requiring a retake to render a diagnostic image. Thus, most of the collimator centering errors that were made did not influence the diagnostic quality of the image. In contrast to the current study’s results, Parks et al found that use of the Rinn® Snap-on rectangular collimating device resulted in a statistically higher number of retakes when compared to the other devices tested (ie, snap-a-ray/round, XCP®/BAI paralleling/round, snap-a-ray/rectangular, XCP®/BAI paralleling/rectangular, XCP/BAI paralleling/Rinn® Snap-on, and Precision/rectangular).7 Although Parks et al did not offer an explanation for this finding, the greater number of retakes might be attributed to the attachment of the rectangular Rinn® Snap-on device to the alignment ring. In addition, Parks et al did not provide a description of the aperture opening for the 16-inch FFD rectangular collimator used in his study, which limits a comparison of his findings to the current study.7 Although not evaluated in this study, use of the XCP-ORA® may reduce the number of collimator cuts due to the notched aiming ring. This could be tested in future studies.
Additionally, the current study found that more errors occurred in posterior projections compared to anterior and bitewing projections regardless of the device used. The authors of this study believe that this phenomenon is likely due to the presence of anatomical obstacles (ie, tongue and cheeks) resulting in the lessening of visual confirmation of proper placement regardless of the device used. Parks et al reported that film placement errors were not affected regardless of collimation technique used or operator skill.6
One of the major challenges in dentistry regarding adoption of dose reduction techniques is whether the user feels that the device helps them to achieve diagnostic images with good image quality. The survey data indicated that the majority of subjects liked the enhancement features of the enhanced device and felt that the enhanced device would render a better diagnostic image. Subjects were able to work faster with the enhanced device and reported preference for this device. Zhang et al found that students reported a greater ease of use with the enhanced rectangular device encompassing a magnetic alignment ring as opposed to the freely adjustable universal rectangular collimator.5 However, contrary to this study’s findings, Zhang et al did not see a reduction in time necessary to complete an FMX.5 This study showed improvement in time efficiency (approximately 4 minutes) as well as a reduction of overall errors with the enhanced device regardless of the fact that in both study settings, subjects had no previous experience with the device. The subjects in this study did have prior experience using the XCP-ORA® which may have contributed to a short learning curve for putting the instruments together and using them for radiographic exposures. Thirty-five percent of the study subjects reported an inaccurate or false confirmation of the light and audible enhancement features of the enhanced device (Figure 9). Similarly, Zhang et al found that false signaling was common.5 As a result, operators should be cautioned that false signals may influence negatively accurate alignment of the x-ray position-indicating device (PID).
It appears that the enhanced device’s enhancement features could have played a part in the reduction of collimator centering errors when compared to the universal device. This study found that the newly modified enhanced device produced fewer collimator centering errors than the freely adjustable universal rectangular device. These findings contradict the findings of Zhang et al who used the originally designed (unmodified) enhanced device.5 Zhang reported that use of the original enhanced device produced almost four times the number of collimator centering errors as with the universal device.5 The 35% larger beam area of the modified enhanced device compared to the originally tested prototype may be the reason for this finding. When image quality was assessed, there appeared to be slightly fewer errors with the use of the enhanced device but these errors did not render diagnostically unacceptable radiographs. Thus, the larger collimator area of the enhanced device may have reduced the number of collimator centering errors, but the data showed that the enhanced device did not produce more diagnostically acceptable images and it may have been at the expense of increased patient exposure.
It is important to note that the exposure area of the Rinn® universal device complies with the NCRP stipulation that rectangular collimated beams should not exceed the dimension of the image receptor by more than 2% of the source-to-image-receptor distance (SID) and has been measured as 1% of the SID.8 The Tru-Align™ website suggests that the enhanced collimator “reduces the beam size to a pattern that is only two percent larger than the acquisition device.”9 But according to a previous study, the measured dimensions were reported to be 4% larger than the SID.8 The manufacturer’s claim of a 50% reduction in exposure area compared with a round collimator appears to be overstated compared to the study by Johnson et al.8,9 Johnson et al determined that a 18% reduction in exposure area occurred when the enhanced device was compared to a 6-cm round collimator.8 Similarly, the claim of a 60% reduction in patient dose is not substantiated with actual measurements.8 Thus, the use of the designation “rectangular collimation” has an implicit expectation of compliance with the standard established for it. As a consequence, the description of the enhanced device as rectangular collimator should be described as “non-standard.”
When interpreting the results of this study, it is important to recognize the limitations of the study design. First, the images from the technical performance component of this study were exposed on DXTTR manikins. Tongue movement and patient cooperation, factors that often influence image acceptability, were not able to be factored in when determining the technical performance of the collimators. Thus, the number and types of errors seen with DXTTRs may be different from live patients. Second, only about half of the study population chose to participate in the study. This may have introduced a subject bias. Thus, a comparison of non-participants with study participants would have helped to determine if differences in groups existed. Although comparisons between groups were not done, attempts were made to standardize a minimum competency level for all subjects. For example, all subjects had passed their preclinical competency and participated in two semesters of radiographic clinical practice. Third, technical differences between the two collimators were based on the radiographic performance skills of the subjects. As mentioned, the subjects had minimal clinical experiences with patients. Performance results of the devices may have been different if they were used by experienced clinicians. Presumably, experienced clinicians are more likely to identify and problem-solve incorrect placement of devices. The authors also made the observation that tubehead instability may influence the interlocking nature of the enhanced rectangular device with its magnetic ring. Enhanced device weight (n = 8) and tubehead instability (n = 4) reported by the subjects may have occurred due to weight of the devices. Weighing of the devices revealed that the universal method was heavier than the test method. Another interpretation might be that the subjects were referring to the weight of the magnetic aiming ring used with the enhanced device, which is heavier and bulkier. In addition, the greater collimator length of the universal method may have contributed to the tubehead drift.
This study compared the performance of two rectangular collimated devices that are currently used in dental practice. While devices with enhancement features may be a step in the right direction, what is of utmost importance is the production of quality images while limiting dose to the patient.
Conclusion
To adhere optimally to the ALARA principle, the authors recommend that radiographers use rectangular collimation meeting NCRP specifications for beam limitation when exposing intraoral radiographs. Adherence to best practices of dental professionals by the adoption of rectangular collimation as a standard of care has been slow to evolve. However, growing concern about the link between low doses of ionizing radiation and the long-term and cumulative risks of cancer ensures this transition to be inevitable. The retail market provides choices to dental professionals when upgrading intraoral imaging equipment for rectangular collimation techniques, thus it is a goal of the authors to promote awareness that all rectangular collimators are not created equally. The rectangular format of a collimator is not by itself sufficient criteria to ensure that a reduction in radiation dose will result when compared to circular collimation. It is pertinent that device manufacturers adhere to guidelines set forth by the NCRP with respect to rectangular collimator dimensions. If the radiographer feels that the presence of enhancement features help them to expose diagnostic images, then the enhanced collimator evaluated in the current study is a better choice than a standard round collimator.
Ultimately, emphasis should be placed on quality training and consistent continuing education to reinforce the techniques and skills involved in imaging optimal intraoral projections. Implementing these recommendations will help to ensure that ionizing radiation is used safely in dental practice and optimal image generation is achieved.
About the Author
K. Brandon Johnson, RDH, MS, is a clinical assistant professor in the Department of Diagnostic Sciences, School of Dentistry. Sally M Mauriello, RDH, EdD, is a professor in the Department of Dental Ecology. John B. Ludlow, DDS, MS, is a professor. Enrique Platin, RT, EdD, is a clinical professor, Department of Diagnostic Sciences. All are at University of North Carolina at Chapel Hill.
Acknowledgments
Authors of the study would like to thank IDI X-Ray for the donation of a Tru-Align™ Rectangular Device for the purpose of this study.
References
1. Ludlow JB, Davies-Ludlow LE, White SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. J Am Dent Assoc. 2008;139(9):1237-1243.
2. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37(2-4):1-332.
3. NCRP Report No. 145, Radiation Protection in Dentistry. Bethesda (MD): National Council on Radiation Protection & Measurements; 2003.
4. American Dental Association Council on Scientific Affairs. The use of dental radiographs: update and recommendations. J Am Dent Assoc. 2006;137(9):1304-1312.
5. Zhang W, Abramovitch K, Thames W, et al. Comparison of the efficacy and technical accuracy of different rectangular collimators for intraoral radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(1):e22-e28
6. Horner K, Hirschmann PN. Dose reduction in dental radiography. J Dent. 1990;18(4):171-184.
7. Parks ET. Errors generated with the use of rectangular collimation. Oral Surg Oral Med Oral Pathol. 1991;71(4):509-513.
8. Johnson KB, Ludlow JB, Mauriello SM, Platin E. Reducing the risk of intraoral radiographic imaging with collimation and thyroid shielding. Gen Dent. 2014;62(4):34-40.
9. Tru-Align™ Frequently Asked Questions [Internet]. Marietta (GA): Interactive Diagnostic Imaging, LLC; c 2011-2015 [cited 2014 May 24]. Available from: http://www.idixray.com/trualign/faq/.