You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Data supporting the steady rise of diabetes in America is indisputable and worrisome. Research estimates conclude that nearly 8% of the US population has diabetes, and another 57 million Americans have pre-diabetes, an altered state of glucose metabolism.1 As the American public’s waistline increases, so do risk factors for developing type 2 diabetes mellitus (T2DM) and other dangerous metabolic diseases. At the same time, researchers note an increased incidence of Alzheimer’s disease. As research continues to uncover a growing number of suspected risk factors characteristic of both diseases, the question arises, is AD actually a form of diabetes?
Research on the correlation between diabetes and AD dates back to the late 1990s. The 1998 Rotterdam Study on incidence and risk of dementia mentions a potential relationship between AD and insulin resistance.2 This study identified a disturbing pattern: a high percentage of the elderly subjects studied who developed AD were patients with T2DM, with double the risk of developing dementia.2 A diabetes prevalence study of elderly patients demonstrated a high incidence of diabetes (nearly 33% of subjects) and impaired fasting glucose (75%), with patients who took insulin demonstrating an even higher risk of developing dementia.3 Another Swedish study in 2008 showed similar rates of increased risk for AD strongly related to midlife development of T2DM.4
Since the early 2000s, researchers focused on the relationship between these two disease processes in hopes of finding early detection methods, effective interventions, and preventive strategies. Common to these studies is the fact that most of the researchers were able to link elevated body mass index, adiposity, impaired fasting glucose, and diabetes to substantially increased risk for AD.5 This growing body of evidence supports the hypothesis that AD is actually a metabolic disease characterized by neurodegeneration and mediated by the impairment of glucose utilization and energy production.3
The term “Type III Diabetes” began to appear in research literature around 2005, when a group of researchers from Brown University examined post-mortem brain tissue samples of patients who had had AD.3 The team of researchers discovered that AD may actually be a neuroendocrine disease associated with insulin signaling. The Brown University researchers chose the term “Type III Diabetes” due to research findings that suggested that AD harbors elements of both type 1 diabetes and type 2 diabetes, namely a decrease in the production of insulin coupled with resistance to insulin receptors.4 De la Monte et al discovered that the post-mortem samples from patients with AD had 80% fewer brain insulin receptors than those in normal subjects.3
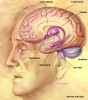
To better understand the risk factors shared by AD and diabetes, it is important to comprehend the relationship of insulin to the brain (Figure 1 through Figure 4). Insulin receptors are widely and irregularly distributed throughout the central nervous system, but are concentrated in the brain. Areas of the brain with the highest concentrations of receptors are the olfactory bulb, pre-frontal cerebral cortex, hypothalamus, cerebellum, and choroid plexus—all areas associated with memory and logic.6 Insulin is able to pass the blood-brain barrier and participates in neurologic function by stimulating enzymes key to the production of acetylcholine, a neurotransmitter necessary and responsible for cognition and memory formation.6 Depleted levels of acetylcholine, while not directly causative, damage cognition by interfering with attentional processing.7 Because research has demonstrated that there are more insulin receptors in the cognition-pertinent areas of the brain, it would seem a logical conclusion that there is a direct association between insulin and cognitive processing.4
Research has consistently demonstrated that when hyperglycemia is present in the brain, insulin resistance becomes a major risk factor for AD disease development and progression.8 Investigators believe this is due to hyperglycemia’s role in promoting the oxidative stress that releases neurotoxins.1 Cumulative oxidative damage is thought to manifest itself metabolically by decreasing acetylcholine production, compromising the endothelial lining tissues of cerebral blood vessels and encouraging formation of deleterious beta-amyloid plaques.7 Furthermore, a recent Tulane University study demonstrated that hyperglycemia linked with T2DM appears to make the beta-amyloid proteins dramatically more toxic to cells lining blood vessels in the brain.9
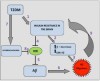
Commonly accepted understanding of disease progression in AD is based on research into the accumulation of neurofibrillary plaque tangles among brain neurons. These tangles result from the overproduction of beta-amyloid proteins that the body is increasingly unable to remove.9 The beta-amyloid plaques have a recognized role in creating neurologic tangles that essentially kill neurons, thereby decreasing cognitive function.4 Additional post-mortem studies have also shown an increased amount of neurofibrillary tangles and beta-amyloid plaques in the hippocampus of patients with diabetes.1
Like the brain, the pancreas can also fall victim to forming beta-amyloid plaques that can ultimately choke the production of insulin by beta islet cells.4 Furthermore, the presence of beta-amyloid plaques in the brain and pancreatic islet cells is a pathogenic feature shared by AD and T2DM.10 De la Monte et al found strong evidence to suggest that the human brain becomes insulin-resistant first, often years before the pancreas demonstrates similar warning signs through A1C, triglyceride, and other blood testing.5 This conclusion by researchers would certainly shed light on why early symptoms of AD can go unnoticed for years.11 Given that researchers are drawing clearer conclusions about AD being associated with hyperglycemia, it is of paramount importance to find reliable and consistent tools for early diagnosis of diabetes.12
Nutritional counseling and medications currently offer only limited health benefits for patients with AD. Traditional cholinomimetic medications such as Aricept® help patients with early AD improve memory loss through increasing levels of available acetylcholine. However, most medications’ effects appear to last only for six to 12 months.6 Another strategy that appears to help is the intranasal administration of insulin.5 Because of its direct entry into nasal passages, the insulin is able to enter the brain quickly via the olfactory and trigeminal channels.1 Metformin, an antilipedemic, is helpful in improving insulin sensitivity and peripheral uptake while decreasing gastrointestinal absorption of glucose.6
It is incumbent on oral healthcare providers to define their role in assisting with early detection of insulin resistance. One recommendation is to consider the advantages of implementing chairside glucose monitoring as an essential vital sign during dental and medical risk assessment. Because many patients visit their dental office more regularly than their physician, the dental team is well poised on the front line for early detection of insulin resistance and for referring patients to their physician.
About the Author
Barbara Graham Hammaker, CRDH, BASDH, MHS, holds a master’s degree in health science from Nova Southeastern University, and a bachelor of applied science in dental hygiene from St. Petersburg College. She has over 34 years of private practice experience in South Florida, and currently works full time as lead instructor and program manager of Dental Hygiene at Broward College in Davie, Florida.
References
1. Akter K, Lanza EA, Martin SA, et al. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71(3):365-376.
2. Ott A, Breteler M, van Harskamp F, et al. Incidence and risk of dementia: the Rotterdam study. Am J Epidemiol. 1998;47(6):574-580.
3. de la Monte SM, Tong M, Wands JR. Insulin resistance, cognitive impairment and neurodegeneration: roles of nitrosamine exposure, diet and lifestyles. In: de la Monte SM (ed): Alzheimer’s disease pathogenesis: care, concepts, shifting paradigms and therapeutic targets. 2011. Available at: www.intechopen.com/books/alzheimer-s-disease-pathogenesis-core-concepts-shifting-paradigms-and-therapeutic-targets.
4. Kroner Z. The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Alt Med Rev. 2009;14(4): 373-379.
5. de la Monte SM. Insulin resistance and Alzheimer’s disease. Biochem Mol Biol Rep. 2009;42(8):475-481.
6. Abdul-Rahman O, Sasvari-Szekely M, Ver A, et al. Altered gene expression profiles in the hippocampus and prefrontal cortex of type 2 diabetic rats. BMC Genomics. 2012;13(81):1-9.
7. Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137-147.
8. Park SA. A common pathogenic mechanism linking type-2 diabetes and Alzheimer’s disease: evidence from animal models. J Clin Neurol. 2011;7(1):10-18.
9. Carvalho C, Katz PS, Dutta S, et al. Increased susceptibility to amyloid beta toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. J Alzheimer’s Dis. 2014;38(1):75-83.
10. de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes — evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101-1113.
11. Sima A, Li Z. Diabetes and Alzheimer’s disease — is there a connection? Rev Diabet Stud. 2006;3(4):161-168.
12. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19-39.