You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
As life expectancy continues to increase, so will the risk of developing a cancer such as Multiple Myeloma (MM). With many people surviving well into their seventh and eighth decades of life, it is very important to understand the unique and complex medical conditions that arise and impact oral health. MM is a hematologic malignancy that may not be well known among dental professionals, and is often characterized by an abnormal proliferation of plasma cells found in the bone marrow. Active proliferation of malignant plasma cells is a strong adverse prognostic factor in multiple myeloma.1 The accumulation of these cells in multiple anatomical sites gives rise to the name MM. Various methods are used in diagnosing MM, such as bone marrow biopsy, fluorescent in situ hybridization, cytogenetics, electrophoresis, quantitative immunoglobulin and radiographic images of the spine, skull and long bones. According to the National Cancer Institute (NCI), MM is staged by estimating the myeloma tumor cell mass on the basis of the amount of monoclonal myeloma protein (M protein) in the serum and/or urine, along with various clinical parameters, such as hemoglobin and serum calcium concentrations, the number of lytic bone lesions, and the presence or absence of renal failure.2 Additionally, imaging of the head/neck may be a vital step in the staging of this disease.
Annually, 4 in every 100,000 people are diagnosed with MM in the U.S.3 Studies have shown that every race is at risk of developing MM, however, African Americans are at a higher risk. MM is more prevalent among males than females and diagnosis occurs most among adults age 65 and older. However, etiology of MM is unknown. Many factors have been associated with its etiology, such as age, gender, race, family history, radiation exposure, obesity and other plasma cell diseases.2 The purpose of this article is to present the clinical course of a patient diagnosed with MM in 2009 who later developed oral complications secondary to the use of a bisphosphonate (BP) during the maintenance phase of his medical treatment.
Case Report
Like many patients presenting with MM, the chief complaint for this patient was severe back pain. Radiographic imaging (MRI) revealed significant bone disease with multiple compression fractures and deformity of the back. The patient attributed his pain to a fall from his motorcycle. He underwent medical intervention at a local spine center in May 2009. An MRI was performed to determine the cause of the patient’s pain. Results at that time revealed no signs of MM. The pain eventually progressed and he began experiencing chest pain. He was admitted to a community hospital in June 2009, where he was diagnosed with pneumonia and a CT scan was done which revealed lytic lesions of the bone, specifically the ribs. Additional hematologic workup showed an increased total myeloma protein (M–protein) in the serum. At the time of this hospitalization, his symptoms included coughing and shortness of breath. In addition to pneumonia his MM specific diagnosis was IgA, lambda. The patient underwent 4 cycles of induction chemotherapy with his private oncologist and was referred to the Greenebaum Cancer Center (a tertiary care facility) for evaluation of possible autologous stem cell transplant in August 2009. After 3 months of treatment his response to therapy was measured. The patient had a very good response, thus he was a candidate for autologous stem cell transplantation. On October 21, 2009, the patient was admitted to the Greenebaum Cancer Center for autologous stem cell transplant. At this time he was treated with additional chemotherapy consisting of Melphalan 200 mg/M2 followed by reinfusion of his previously harvested stem cells. The patient remained hospitalized for 14 days and was discharged as his hematologic status recovered.
Prior to initiation of autologous stem cell transplantation, the patient was evaluated by the Oral Medicine service of the Greenebaum Cancer Center in August 2009. A review of his past medical history included tobacco abuse in the form of cigarettes (1 pack per day, for 5 years), which he quit using in 1980. He is a social drinker and had occupational exposure to automobile chemicals (engine oil, transmission fluid, brake fluid, power steering fluid and engine coolant) from his years of working as an automobile mechanic. Prior to stem cell transplantation, the patient received a single infusion with zoledronic acid (Zometa®; Novartis Pharmaceuticals Corp., East Hanover, NJ). Post stem cell transplant, he has received zoledronic acid monthly for a total of 2 years of BP therapy. During this time he received an oral assessment every 3 months while undergoing care at the Greenebaum Cancer Center. Dental hygiene instrumentation was not performed during the treatment of the bisphosphonate– associated osteonecrosis of the jaw (BON) lesion.
An oral assessment, including soft and hard tissue examination, prior to autologous peripheral blood stem cell transplant was completed in August 2009 and revealed multiple missing teeth (1, 3, 14, 15, 19, 20, 21, 30 and 32) as well as multiple restorations. His dentition was in fair to poor condition and he was at a high risk for dental caries due to inadequate biofilm control, inadequate fluoride use, high fermentable carbohydrate intake and dry mouth. Professionally applied topical fluoride, in the form of gel or varnish, is recommended at 3 to 6 months intervals for patients with increased caries risk.4 There was moderate supra and subgingival calculus present. Generalized recession also increased his risk of developing root caries. Oral care included brushing once a day with a manual toothbrush and infrequent use of floss. Probing depths revealed generalized 4 to 6 mm pocketing. His periodontal status was classified as moderate to severe, uncontrolled periodontitis with mild to moderate bleeding on probing.
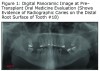
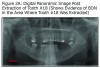
The initial panoramic radiograph exposed on August 5, 2009 revealed evidence of caries on the distal of #18 (Figure 1). Treatment options including root canal therapy or extraction were discussed with the patient. For the prevention and management of BON in patients at risk, the American Academy of Oral Medicine has developed clinical guidelines.5 The patient was informed of the risk of developing BON post extraction. After considering the risks the patient elected extraction of #18; this was accomplished by his private dentist in September 2009, prior to stem cell transplant. Panoramic radiographs reveal the status of tooth #18 before the extraction (Figure 1) and an area of bony changes about 18 months after the extraction (Figure 2A, Figure 2B).
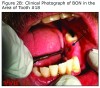
During one of his routine quarterly oral evaluations on March 3, 2011, an area of exposed bone was visible in the left mandible at the extraction site of #18. The patient was asymptomatic. Antibiotic therapy was initiated. In June 2011, after 12 weeks of antibiotic therapy, including generic amoxicillin (Sandoz Inc., Princeton, NJ) 875 mg, twice daily and generic chlorhexidine oral rinse, 20 ml x30 second’s, twice daily, there was no clinical improvement. The site appeared to have increased (6x9 mm) in dimension; however, the patient remained asymptomatic.
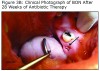
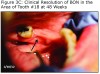
An evaluation of the site on September 27, 2011 showed that the area of BON had not healed and the buccal tissue adjacent to the exposed bone appeared enlarged. The antibiotic regimen was changed to include Augmentin® 875 mg (Sandoz Inc. Princeton, NJ) plus 500 mg metronidazole (Flagyl®; Teva Pharmaceuticals, Wales, Penn) to be taken twice daily. Prognosis for the BON lesion was guarded. The necrotic bone in this area started to separate from the mandible following re–epithelization below the bone (Figure 3A) and at 6 months (Figure 3B) shows the lesion was healing. At 7 months the necrotic bone was easily removed by the Greenebaum Cancer Center’s attending dentist and the site was left to heal by secondary intention. A clinical photograph (Figure 3C) at 48 weeks of treatment with antibiotics and antimicrobial mouth rinse revealed resolution of BON. Antibiotics and antimicrobial mouth rinse were discontinued. Currently, the patient continues to receive Zometa infusion quarterly as part of his MM management. Following lesion resolution the patient has received routine dental care including dental hygiene instrumentation with his private dentist.
Discussion
Bone pain, broken bones, weakness, weight loss, fatigue and repeated infections may be signs and symptoms of MM.6 Oral manifestations such as gingival hemorrhage, odontalgia, paresthesias, tooth mobility and ulcerations may also be present.7 Referral to a dental professional for comprehensive oral evaluation is important prior to initiation of medical treatment and if oral complications develop during medical treatment.
People with MM are living longer which means an increased opportunity for these survivors to present as patients in private dental practices. Increasing survivorship resulting from improved management of MM is partially attributed to the development of new supportive care measures, i.e. medications. BPs are a newer class of drugs utilized in the treatment of many patients with MM and other cancers where metastatic bone disease is present. It works by preventing bone osteoclastic resorption while also increasing bone density. Treatment of MM with BP may result in oral sequelae such as BON. BON is defined by the American Association of Oral and Maxillofacial Surgeons as the presence of exposed bone persisting for more than 8 weeks in the oral cavity of an individual treated with a BP with no prior history of radiation to the head or neck tissues.8 BON closely resembles the occupational disorder historically referred to as “fossy jaw,” whereby workers in match production factories were exposed to white phosphorous during the manufacturing process.9 A retrospective study conducted by Badros et al evaluated 90 patients diagnosed with MM, and described the medical and dental characteristics of 22 of these patients who developed BON while undergoing intravenous (IV) BP therapy. The authors concluded that BON is an emerging problem in MM patients which affects older patients with myeloma who had received long–term BP therapy.10 BP is administered either through IV or oral routes, hence creating 2 separate classes of BPs. Patients receiving IV BP are more likely to develop BON than those taking oral BP. The predicted risk of developing BON due to IV BP is 0.16 times the odds of those not receiving IV BP.10 Table I summarizes current IV BPs in use today.
Oral manifestations of MM and/or its medical management can only be identified by thorough surveillance of this patient population. Two studies, 1 retrospective and 1 cross–sectional study using 2 different populations were conducted in 2 German neighboring cities with the intention of identifying the prevalence of BON in individuals with MM and comparing it with existing published data. The authors assessed the occurrence of oral manifestations among participants in both studies. They concluded that the prevalence of BON may have been underestimated to date. BON was recorded for nearly 5% (4/81) of the subjects in the retrospective study and nearly 21% (16/78) in the cross–sectional study. An oral examination of all patients in the cross–sectional portion of the study might explain the higher prevalence. Since nearly all patients with BON had an additional trigger factor (previous extraction, surgical dental procedure and the use of chemotherapy and corticosteroid). Routine oral hygiene and dental care might help to reduce BON incidence, especially prior to BP administration.11
The number of patients with MM that will develop BON after a period of receiving BP is unknown. Clinically, the reported incidence of BON ranges from 1.3% to 21%, with a higher frequency in the mandible than in the maxilla.10,12–19 BON occurs in a dose and time dependent manner, with cases being more prevalent in those on IV dosing and for time periods of 10 to 59 months10,13–17 As in this case, it is most often associated with a dental procedure or trauma, however, many are shown to occur spontaneously.8,20 The microbial aspects of BON have focused on the uniqueness of the oral cavity where continuous host–microbe interactions take place, leaving behind bacterial smear layers and potentially inducing microenvironment acidosis.8,20 Additionally, the jaws are a site of constant loading and unloading of the bone underlying an exquisitely thin mucosal layer producing constant bone remodeling attempts and BP accumulation.21 These unique characteristics of the oral cavity allow for bony exposure, microbial colonial expansion, free BP release and an acidic microenvironment, all of which permit the process to increase bone necrosis, decrease bone regeneration and inhibit healing of soft tissue.21–24
Until recently, all of the cases of osteonecrosis of the jaw have been associated with BP therapy. However, new cases are emerging associated with anti–resorptive therapies. These drugs include Denosumab, a monoclonal antibody that selectively binds receptor activator of nuclear factor kappa–B ligand or RANKL, and Bevacizumab, a monoclonal antibody that inhibits angiogenesis through vascular endothelial growth factor (VEGF–A).25–29 The American Dental Association recently suggested that this clinical finding be described as anti–resorptive osteonecrosis of the jaw or ARONJ, thus including both BP and non–BP drugs.30 Although these drug–induced lesions appear clinically similar to BON lesions, their developmental mechanism is currently unknown. Further studies are necessary to fully elucidate the pathophysiology of oral lesions secondary to anti–resorptive drugs.
Anyone with poor oral habits who is taking or has taken a BP is at risk of developing BON. Recommendations to minimize the potential for BON in MM patients include maintaining good oral hygiene and frequent oral health assessment. Individuals receiving Zometa are encouraged to have a pre–treatment oral health assessment with a dentist/dental hygienist with the goal being completion of necessary dental work prior to initiation of BP.31,32 Dental professionals should evaluate the risk associated with chronic dental disease while providing dental treatment to individuals receiving long term BP therapy for MM. Published treatment strategies for BON depend on the stage of the lesion. Patients without evidence of necrotic bone require no treatment, however, patient education is necessary regarding future risk of developing BON. Antibacterial mouth rinse and quarterly clinical follow–up are recommended to treat asymptomatic exposed and necrotic bone without evidence of infection. Symptomatic exposed necrotic bone treatment recommendation includes antibiotic, oral antibacterial mouth rinse, anesthetics, superficial and or surgical debridement. Though there may be cases that do not respond to these treatments, the American Association of Oral Maxillofacial Surgeons and the American Academy of Oral Medicine have provided these recommendations as guidelines to be used by dental and dental hygiene professionals in the management of BON.8,11 A multidisciplinary management approach is recommended to ensure optimum treatment and to minimize the risk of BON.
A healthy dentition and an oral cavity free of infection will help reduce the risk of BON. Once established, BON may persist for months or years and treatment is symptomatic in nature. It is important to rule out other medical and/or dental conditions when involved in the management of MM patients. Misdiagnosis may include alveolar osteitis, osteomyelitis and osteoradionecrosis, and symptoms may mimic sinusitis, gingivitis/periodontitis, periapical pathology and temporomandibular joint disorder.33
Conclusion
As practicing clinicians involved in oral assessment, dental hygienists are poised to identify and manage the oral health risks of MM patients undergoing systemic treatment. Providing patients with current information related to the risk/benefit of invasive dental treatment in the setting of bisphosphonate and anti–resorptive therapy is a responsibility of the dental hygienist. Collaboration with other disciplines in the treatment of the MM patient is essential in treatment planning and coordinating recommended dental care. The development of BON may lead to delays or adjustments in medical care and/or dental care, either of which may be detrimental to the patient’s overall health.
Melvin J. Teah, RDH, BS, works in private practice in Baltimore. Sheryl L. Ernest Syme, RDH, MS, is an associate professor, Division of Dental Hygiene, Department of Periodontics, University of Maryland School of Dentistry, Baltimore. Mark Scheper, DDS, PhD, works in private practice in Baltimore. Dianna S. Weikel, RDH, MS, is an associate professor, Department of Oncology and Diagnostic Sciences, University of Maryland School of Dentistry and Greenebaum Cancer Center, Baltimore.
References
1. Hose D, Rème T, Hielscher T, et al. Proliferation is a central independent prognostic factor and target for personalized and risk–adapted treatment in multiple myeloma. Haematologica. 2011;96(1):87–95.
2. Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment: Health Professional Version. National Cancer Institute [Internet]. [cited 2011 April 4]. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/myeloma/Patient
3. Kyle RA. Plasma cell disorder: Hematologic Diseases. In: Goldman L, Bennett CL. Cecil Textbook of Medicine. 21st ed. Philadelphia: WB Saunders Company; 2000. p. 977–980.
4. American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: Evidence–based clinical recommendations. J Am Dent Assoc. 2006;137(8):1151–1159.
5. Walter C, Al–Nawas B, Frickhofen N, et al. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med. 2010;6:11.
6. What you need to know about multiple myeloma. National Cancer Institute [Internet]. [cited 2008 Nov 16]. Available from: http://www.cancer.gov/cancertopics/wyntk/myeloma
7. Vieira–Leite–Segundo A, Lima Falcão MF, Correia–Lins Filho R, Marques Soares MS, López López J, Chimenos Küstner E. Multiple Myeloma with primary manifestation in the mandible: A case report. Med Oral Patol Oral Cir Bucal. 2008;13(4):232–234.
8. Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate–related osteonecrosis of the jaws––2009 update. J Oral Maxillofac Surg. 2009;67(5):2–12.
9. Pickett FA, American Academy of Oral Medicine. Bisphosphonate–associated osteonecrosis of the jaw: A literature review and clinical practice guidelines. J Dent Hyg. 2006;80(3):10.
10. Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J Clin Oncol. 2006:24(6):945–952.
11. Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate–associated osteonecrosis. An American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136(12):1658–1668.
12. Walter C, Al–Nawas B, Grötz KA, et al. Prevalence and risk factors of bisphosphonate–associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol. 2008;54(5):1066–1072.
13. Bagan JV, Jimenez Y, Murillo J, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases. Oral Oncol. 2006;42(3):327–329.
14. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–8587
15. Mavrokokki T, Cheng A, Stien B, Goss A. Nature and frequency of bisphosphonate–associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3):415–423
16. Woo SB, Hellstein JW, Kalmar JR: Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761.
17. Aragon–Ching JB, Ning YM, Chen CC, et al. Higher incidence of osteonecrosis of the jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti–angiogenic agents. Cancer Invest. 2009;27(2):221–226.
18. Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate–induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44(9):857–869.
19. Advisory Task Force on Bisphosphonate–Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate–related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–376.20. Borrás–Blasco J, Rosique–Robles D, Giner–Marco V, Galan–Brotons A, Casterá E, Costa S. Possible delayed onset of osteonecrosis of the jaw in association with zoledronic acid. A case report. J Clin Pharm Ther. 2007;32(6):651–654.
21. Goffinet M, Thoulouzan M, Pradines A, et al. Zoledronic acid treatment impairs protein geranyl–geranylation for biological effects in prostatic cells. BMC Cancer. 2006;6:60.
22. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen–containing bisphosphonates inhibit the mevalonate pathway and prevent post–translational prenylation of GTP–binding proteins, including ras. J Bone Miner Res. 1998;13(4):581–589
23. Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66(4):767–775.
24. Weitzman R, Sauter N, Eriksen EF, et al. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients––May 2006. Crit Rev Oncol Hematol. 2007;62(2):148–152.
25. Taylor KH, Middlefell LS, Mizen KD. Osteonecrosis of the jaws induced by anti–RANK ligand therapy. Br J Oral Maxillofac Surg. 48(3):221–223.
26. Kyrgidis A, Toulis KA. Denosumab–related osteonecrosis of the jaws. Osteoporos Int. 2011;22(1):369–370.
27. Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on Denosumb. J Oral Maxillofac Surg. 2010;68(5):959–963.
28. Migliorati CA, Covington JS 3rd. New oncology drugs and osteonecrosis of the jaw (ONJ). J Tenn Dent Assoc. 2009;89(4):36–38.
29. Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26(24):4037–4038.
30. Hellstein JW, Adler RA, Edwards B, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142(11):1243–1251.
31. Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137–145.
32. Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117–120.
33. Khosla S, Burr D, Cauley J, et al. Bisphosphonate–Associated Osteonecrosis of the Jaw: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491.