You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Dental implants have provided a highly predictable means of replacing missing or soon to be missing teeth.1-3 Their ability to osseointegrate and remain stable while also minimizing the dentistry that might be needed on adjacent teeth has made them the preferred choice to replace missing or hopeless teeth. Nonetheless, long-term observations have identified problems with dental implants that were not necessarily apparent or reported on in the early years of their implementation. Biologic complications of dental implants, including peri-implant mucositis and peri-implantitis, have received considerable attention. Peri-implantitis is the most severe of these complications, occurring when crestal bone loss extends beyond what typically would be expected with normal physiologic modeling/remodeling, and, when ongoing, it inevitably can lead to the loss of the dental implant(s).4-6
While myriad treatment methods have been proposed for the management of peri-implantitis, there is no consensus as to the best way to approach this problem.7 A review of the literature indicates that anti-infective approaches have traditionally been attempted for the eradication of peri-implantitis, with efforts made to remove dysbiotic bacterial biofilm.8-14 Nonsurgical methods typically have been ineffective, most likely because of an inability to access and properly decontaminate the implant surface.15,16 Open-flap approaches also have been advocated, both with and without various surface decontamination options and regenerative protocols.13,14 While some of these surgical access approaches have been successful, achieving a favorable outcome can be challenging for several reasons. Access can be difficult given the depth of some lesions and the narrowness of the intrabony component, hindering the ability to properly clean/decontaminate the implant's entire surface.17 Hence, managing the intricate microgeometry of many of these roughened surfaces is virtually impossible. Many implants have their surfaces located outside an infrabony defect that extends beyond the buccal or lingual housing of bone.18,19 This may relate partly to the implant's location upon placement or perhaps be due to ongoing craniofacial growth and development. In these instances, use of a regenerative or flap-only approach without surface modification may fail to arrest the disease and leave the implant threads and roughened surface exposed.14
Resective approaches are another method used to treat peri-implantitis. It is generally believed among clinicians that the instruments used to clean the outer surface of a dental implant are inadequate as they cannot render it free of plaque, calculus, and endotoxin, and there are no studies that closely address the efficacy of plaque removal from the surface by such instruments (eg, scalers). To better accomplish this endeavor, high-speed handpieces may be used to remove the outer affected surface, leaving the implant in a state that more approximates the micro-roughness of the original machined implant. Romeo and colleagues were among the first to report on the success of implantoplasty with 3-year follow-up attesting to its success for halting peri-implantitis.20,21 They performed the implantoplasty of the surface in conjunction with resective therapy, and there was no indication of any intrabony lesion present or left after treatment. Schwarz et al advocated the use of implantoplasty but only for the supracrestal component, as a part of comprehensive management of the dental implant.22 The purpose of the implantoplasty is to achieve a highly polished surface that will limit plaque adherence and help facilitate the patient's oral hygiene efforts. This algorithm also includes soft-tissue grafting and regenerative therapy. Any infraosseous component of the implant that will be grafted receives mechanical surface detoxification,23 which may include the use of curettes, ultrasonic instruments, titanium or nickel-titanium brushes, or air-abrasive devices. After the visible calculus and biofilm have been removed, chemical detoxification is carried out using citric acid, hydrogen peroxide, or ethylenediaminetetraacetic acid (EDTA).
Esteves Lima et al recently performed an exhaustive systematic literature review and meta-analysis on implantoplasty use and determined that some evidence exists to recommend it as a potential treatment for peri-implantitis.24 Given the complex morphology of many peri-implantitis lesions, there is a need for a combined approach where both resection and regeneration are performed.22 Because implantoplasty can provide an endpoint where the affected dental implant surface can be more easily cleansed through supportive maintenance efforts as well as provide a surface for both soft- and hard-tissue adherence, this procedure might be an optimal method for treating peri-implantitis. The dental implant surface in the subcrestal portion needs to be thoroughly decontaminated and achieve a matte-like surface without being overly smooth, because a highly polished endpoint is less desirable for bone-to-implant contact.25
The following case reports are presented in which implantoplasty that employed 12- and 30-fluted carbide surgical-length finishing burs was used to decontaminate and smooth both the supra- and subcrestal portions of the dental implant's surface. Verification that this might be an excellent method for subcrestal surface decontamination is provided in the radiographic and re-entry evidence suggesting favorable bone gain at 12 months and 10 months, respectively.
Case Reports
Case 1
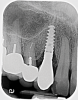
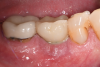
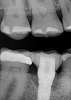
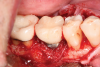
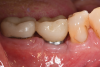
A 56-year-old woman was referred for the treatment of a dental implant at the site of the maxillary right canine, No. 6, which was diagnosed with moderate peri-implantitis subcategory A (Figure 1).19 The implant had been placed 3 years prior and had healed uneventfully, but at a recent maintenance visit purulence and pocket depth of up to 8 mm were noted. The patient's medical history included hypothyroidism, for which she took levothyroxine, and an allergy to sulfa medications. She had a high smile line, and the dental implant was 3 mm wide with an anodized surface.
The author (PSR) administered initial treatment with a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. This therapy, however, demonstrated no improvement by 6 months as 8 mm depths with bleeding remained (Figure 2).
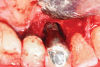
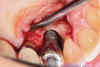
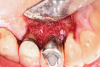
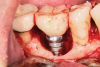
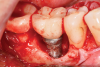
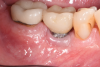
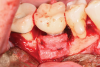
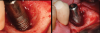
A surgical approach was planned that included implantoplasty and guided bone regeneration. After local anesthesia was administered, full-thickness flaps were elevated using an envelope flap on the buccal aspect and a triangular design on the palate to expose the defects. Removal of granulomatous tissue from the area enabled visualization of a narrow circumferential lesion on the facial (Figure 3) and a one- to two-wall combination defect on the palatal (Figure 4). The surface was initially treated with a 50% solution of citric acid for 30 seconds followed by thorough rinsing with sterile saline. This was followed by implantoplasty using a 30-fluted finishing bur of surgical length. Because the implant was very narrow, achieving a matte-like surface was not possible; thus, the goal was to reduce the threads and lightly remove the implant's outer surface to expose fresh titanium (Figure 5 and Figure 6). The modified surface was treated again with citric acid and rinsed with sterile saline.
A composite allograft was placed into the defect that contained cryopreserved demineralized freeze-dried bone fibers with cancellous bone chips containing mesenchymal stem cells (Figure 7). This was layered with a dehydrated human de-epithelialized amnion-chorion membrane. The flaps were repositioned and secured with 5-0 polytetrafluoroethylene (PTFE) sutures using an interrupted technique.
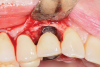
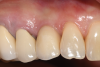
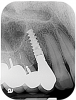
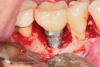
For infection control, the patient was placed on amoxicillin for the first week and told to rinse with a phenolic oral mouthrinse for the first 3 months. The patient was instructed to refrain from mechanical oral hygiene efforts for the first month. She underwent stringent postoperative management for the first year. Clinical examination 1 year postoperatively demonstrated that the 8 mm to 9 mm probing depths on the mesial and distal palate now measured 4 mm without bleeding (Figure 8), and a periapical radiograph suggested favorable bone gain (Figure 9).
Case 2
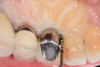
A 66-year-old man was referred for evaluation and treatment of a dental implant at the site of the mandibular right first molar, No. 30, which had been placed and restored 9 years prior. The patient's dentist had noted bone loss at the implant at the patient's recent semiannual maintenance visit. The patient's medical history included hypertension, for which losartan and amlodopine were being taken, and elevated cholesterol, for which rosuvastatin was being taken.
Based on clinical and radiographic examination of the area, the patient was diagnosed with moderate peri-implantitis subcategory A (Figure 10 and Figure 11).19 Pocket depths at this implant ranged up to 9 mm. A surgical approach was planned that included implantoplasty and guided bone regeneration.
After local anesthesia was administered, full-thickness flaps were elevated from the buccal and lingual aspects of tooth No. 27 to the distobuccal and lingual aspects of tooth No. 31. Removal of granulomatous tissue from the implant site allowed visualization of a combination lesion with two walls at the distal and two to three walls at the mesial with a circumferential configuration on the lingual (Figure 12). The surface was initially treated with a 50% solution of citric acid for 30 seconds, which was burnished on with cotton pellets followed by thorough rinsing with sterile saline. This was followed by implantoplasty using 12-fluted followed by 30-fluted surgical-length finishing burs that provided access to the base of the defect. Because the crown was cemented, which obviated access, a matte-like surface could not be achieved; thus, the goal was to reduce the threads and remove the outer affected surface to expose fresh titanium (Figure 13). At this stage, the implant was wiped again with citric acid and rinsed with sterile saline.
As in the first case, a composite allograft was placed into the defect that contained cryopreserved demineralized freeze-dried bone fibers with cancellous bone chips containing mesenchymal stem cells (Figure 14). This was layered with an amnion-chorion membrane. The flaps were repositioned and secured with 5-0 PTFE sutures using an interrupted technique.
For infection control, the patient was placed on amoxicillin for the first week and instructed to rinse with a phenolic oral mouthrinse for the first 3 months. The patient was told to refrain from mechanical oral hygiene efforts for the first month. He underwent stringent postoperative management for the first 10 months.
At 10 months (Figure 15), it was decided to modify the phenotype by placing acellular dermal matrix (ADM) to increase the soft-tissue thickness. Upon opening of the site, the osseous fill that had occurred in the mesial infrabony aspect could be visualized (Figure 16), and where there was incomplete thread removal at the distal, this area was slightly modified using the implantoplasty burs. The ADM allograft was placed and secured with 5-0 chromic gut suture using an interrupted technique (Figure 17), and the flaps were secured with 5-0 PTFE. At 15 months post-surgery, probing depths ranged up to 4 mm with the absence of bleeding, and the phenotype had been favorably modified (Figure 18).
Discussion
The two cases presented demonstrate the success that can be achieved using implantoplasty to decontaminate the entire dental implant surface. How this approach differs from past endeavors is that the surface modification used in these cases extended to the depth of the patients' infrabony lesions in an effort to achieve reosseointegration. Several case report articles in the literature with human histologic evidence show that regeneration may be an achievable endpoint for implants compromised by peri-implantitis.26-28 While these three case reports utilized different surface management protocols in humans, what they had in common is that the superstructure/crown was removed to attain access for cleaning all aspects of the surface.26-28 Removal of the superstructure/crown, however, is not always possible as some crowns are cemented, and if removed they will require replacement. The benefit of the longer 30-mm length burs in the bur kit used in the present cases is that they allow for better access due to a variety of shapes, reaching even to the subcrestal portions of the defect, which can be obviated by crowns that cannot be removed.29 While this may not prohibit achieving a completely matte-type finish on the implant surface, the fact that the threads and outermost portion get reduced/eliminated would facilitate decontamination.
El Chaar et al used scanning electron microscopy to assess various chemotherapeutic and mechanical methods for surface decontamination of infected dental implants retrieved from humans.30 They noted that chemical agents failed to remove any biologic debris. Airborne-particle abrasion, laser, and titanium brush removed part of the biologic debris, while implantoplasty demonstrated complete biologic debris removal. A study by Htet et al in beagle dogs with ligature-induced peri-implantitis compared a variety of techniques used to decontaminate the surfaces of dental implants with an anodized surface.31 These authors evaluated results through clinical, radiologic, histologic, and histomorphometry and found that the use of a bur and the combined use of a bur plus citric acid achieved endpoints that were equivalent to, if not better than, those of other methods of surface decontamination.
Capitalizing on a highly intricate and complex eight-step methodology for surface decontamination prior to osseous grafting, Froum et al demonstrated successful clinical regenerative results with 2- to 10-year follow-up,32 albeit a small cohort within the group of treated patients in the case series required revisions to maintain their dental implants, and several implants were lost. Despite the ability of this eight-step algorithm to achieve successful decontamination in vitro,33 an aggressive physical approach, much like El Chaar et al noted in their article,30 may be needed to improve decontamination predictability. Some of the suboptimal results in the Froum et al study32 may have been due to difficulty accessing the implant surface, particularly in those bony lesions with a narrower morphology or where the more apical extent of the defects might have been extremely difficult to reach and modify.
Peri-implantitis destruction results in bony defects with a wide variety of morphologies.17 Many of these lesions contain an infrabony component with multiple walls, which would suggest that a regenerative approach be considered as a part of the treatment. Chan and colleagues performed a systematic review and meta-analysis of various surgical approaches for peri-implantitis, including access flap and debridement, surgical resection, application of bone grafting materials, and guided bone regeneration.34 Within the limitation of their systematic review, the authors concluded that application of grafting materials and barrier membranes resulted in greater probing depth reduction and radiographic bone fill. However, they noted that there was a lack of high-quality comparative studies to support this statement. Implantoplasty may be used to achieve a surface absent bacterial contamination in both the supra- and subcrestal components of a peri-implantitis defect and, thus, provide a uniform way to address the entire implant surface. This enables the surgeon to utilize a regenerative approach for the subcrestal area, even in the narrowest of defects, while not forcing the use of resective treatment, which can leave the implant body more exposed to the oral environment. The finishing burs used in the present cases are effective at eliminating/reducing the implant threads and creating a more matte-like implant surface that is not overly smooth, which is desirable because a highly polished endpoint is less suitable for bone-to-implant contact.25,35,36 Removal of the implant crown provides optimal access for thread removal and surface smoothing (Figure 19) and allows for healing within a closed environment.35-37 Use of burs with longer surgical length and myriad shapes provides enhanced access to all aspects of the implant body than what was typically available in the past. Moreover, the use of both 12- and 30-fluted burs is beneficial considering that implants made from grade 5 titanium may require a lesser fluting to aid in reducing the outer surface of the implant, while the 30-fluted burs can help to avoid excessive reduction of narrower-diameter implants, such as seen in Case 1.
Like any technique, implantoplasty has potential shortcomings. First, the purposeful removal of titanium surface and threads for decontamination of the implant may pose a risk for its future fracture. Costa-Berenguer et al reviewed potential fracture following implantoplasty with in vitro mechanical fracture resistance testing for standard-diameter externally hexed dental implants made from grade 4 titanium.38 They noted that, although technically demanding and time-consuming, implantoplasty did not seem to significantly alter fracture resistance of these dental implants due to fatigue. Also, unlike earlier dental implants, which were manufactured from grade 1 commercially pure titanium, current implants are made from grades 3, 4, and 5 titanium or an alloy of titanium and zirconia, which are harder and less prone to fracture.39 Nevertheless, for narrower-diameter implants, much like was used in Case 1, the clinician might consider minimally reducing the implant surface, which may mean diminishing the threads to a flatter topography rather than eliminating them, and only lightly debriding the surface rather than giving it a matte-like finish. Chemotherapy, such as the application of citric acid,32,40 might be considered in combination with the implantoplasty, particularly in the area of regeneration, since a more aggressive implantoplasty is not being performed with a narrower-diameter implant.
Another concern with implantoplasty is the potential for dispersion of titanium granules during the smoothing process. Studies have demonstrated an association with titanium particles and the breakdown of dental implants.41-43 This dispersion not only can be due to such factors as abutment micromotion but can occur during any time throughout the process, including at dental implant insertion.44 Care must be taken to remove as much of the generated particulate matter as possible; one way to do this is to run the implantoplasty burs lightly on the surface of the bone with copious irrigation.
Conclusion
This article demonstrated the use of implantoplasty as a means to decontaminate the surface of a dental implant below the crest of the bone in addition to its historic use supracrestally when performing regenerative therapy. The goal in the two cases presented was to create a surface that would be free of contamination to allow for reosseointegration as well as improved cleansability by both the patient and dental co-therapists. To the authors' knowledge this is the first time in the literature that implantoplasty has been used in this manner and demonstrated potential regenerative efficacy. Future studies are needed to determine the generalizability of this technique for a larger patient cohort provided by a greater number of clinicians.
About the Authors
Paul S. Rosen, DMD, MS
Clinical Professor of Periodontics, Rutgers University School of Dental Medicine,Newark, New Jersey; Private Practices, Yardley, Pennsylvania, and New York, New York
Dennis P. Tarnow, DDS
Clinical Professor of Periodontics and Oral Implantology, Columbia University Collegeof Dental Medicine, New York, New York; Private Practice, New York, New York
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Adell R, Erikkson B, Lekholm U, et al. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5(4):347-359.
2. Buser D, Mericske-Stern R, Bernard JP, et al. Long term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8(3):161-172.
3. Moraschini V, Poubel LA, Ferreira VF, Barboza E. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44(3):377-388.
4. Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005;16(4):440-446.
5. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 suppl):286-291.
6. Koldsland OC, Scheie A, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81(2):231-238.
7. Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29 suppl:325-345.
8. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63-76.
9. Schwarz F, Jepsen S, Obreja K, et al. Surgical therapy of peri-implantitis. Periodontol 2000. 2022;88(1):145-181.
10. Fu JH, Wang HL. Breaking the wave of peri-implantitis. Periodontol 2000. 2020;84(1):145-160.
11. Klinge B, Gustafsson A, Berglundh T. A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J Clin Periodontol. 2002;29 suppl 3:213-225.
12. Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human peri-implantitis microbiota. Clin Oral Implants Res. 2014;25(1):82-90.
13. Leonhardt Å, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol. 2003;74(10):1415-1422.
14. Charalampakis G, Leonhardt Å, Rabe P, Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clin Oral Implants Res. 2012;23(9):1045-1054.
15. Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(8 suppl):305-315.
16. Romanos GE, Weitz D. Therapy of peri-implant diseases. Where is the evidence? J Evid Based Dent Pract. 2012;12(3 suppl):204-208.
17. Monje A, Pons R, Insua A, et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relate Res. 2019;21(4):635-643.
18. Monje A, Galindo-Moreno P, Tözüm TF, et al. Into the paradigm of local factors as contributors for peri-implant disease: short communication. Int J Oral Maxillofac Implants. 2016;31(2):288-292.
19. Rosen PS, Froum SJ, Sarmiento HL, Wadhwani CP. A revised peri-implantitis classification scheme: adding three-dimensional considerations to facilitate prognosis and treatment planning. Int J Periodontics Restorative Dent. 2022;42(3):291-299.
20. Romeo E, Ghisolfi M, Murgolo N, et al. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res. 2005;16(1):9-18.
21. Romeo E, Lops D, Chiapasco M, et al. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: radiographic outcome. Clin Oral Implants Res. 2007;18(2):179-187.
22. Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res. 2014;25(1):132-136.
23. Froum SJ, Dagba AS, Shi Y, et al. Successful surgical protocols in the treatment of peri-implantitis: a narrative review of the literature. Implant Dent. 2016;25(3):416-426.
24. Esteves Lima RP, Abreu LG, Belém FV, et al. Is implantoplasty efficacious at treating peri-implantitis? A systematic review and meta-analysis. J Oral Maxillofac Surg. 2021;79(11):2270-2279.
25. Hermann JS, Jones AA, Bakaeen LG, et al. Influence of a machined collar on crestal bone changes around titanium implants: a histometric study in the canine mandible. J Periodontol. 2011;82(9):1329-1338.
26. Fletcher P, Deluiz D, Tinoco EM, et al. Human histologic evidence of reosseointegration around an implant affected with peri-implantitis following decontamination with sterile saline and antiseptics: a case history report. Int J Periodontics Restorative Dent. 2017;37(4):499-508.
27. Kim S, Hu KS, Jung UW. Reosseointegration after regenerative surgical therapy using a synthetic bone substitute for peri-implantitis: human autopsy study. Int J Periodontics Restorative Dent. 2018;38(4):585-591.
28. Bosshardt DD, Brodbeck UR, Rathe F, et al. Evidence of re-osseointegration after electrolytic cleaning and regenerative therapy of peri-implantitis in humans: a case report with four implants. Clin Oral Investig. 2022;26(4):3735-3746.
29. Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res. 2013;24(1):91-95.
30. El Chaar E, Almogahwi M, Abdalkader K, et al. Decontamination of the infected implant surface: a scanning electron microscope study. Int J Periodontics Restorative Dent. 2020;40(3):395-401.
31. Htet M, Madi M, Zakaria O, et al. Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: lasers and mechanical debridement with citric acid. J Periodontol. 2016;87(8):953-961.
32. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863.
33. Rosen PS, Qari M, Froum SJ, et al. A pilot study on the efficacy of a treatment algorithm to detoxify dental implant surfaces affected by peri-implantitis. Int J Periodontics Restorative Dent. 2018;38(2):261-267.
34. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041.
35. Mordini L, Sun N, Chang N, et al. Peri-implantitis regenerative therapy: a review. Biology (Basel). 2021;10(8):773.
36. Wen SC, Barootchi S, Huang WX, Wang HL. Surgical reconstructive treatment for infraosseous peri-implantitis defects with a submerged healing approach: a prospective controlled study. J Periodontol. 2022;93
(2):195-207.
37. Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Submerged healing following surgical treatment of peri-implantitis: a case series. J Clin Periodontol. 2007;34(8):723-727.
38. Costa-Berenguer X, García-García M, Sánchez-Torres A, et al. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin Oral Implants Res. 2018;29(1):46-54.
39. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Factors influencing the fracture of dental implants. Clin Implant Dent Relat Res. 2018;20(1):58-67.
40. Gamal AY, Abdel-Ghaffar KA, Iacono VJ. A novel approach for enhanced nanoparticle-sized bone substitute adhesion to chemically treated peri-implantitis-affected implant surfaces: an in vitro proof-of-principle study. J Periodontol. 2013;84(2):239-247.
41. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15.
42. Fretwurst T, Buzanich G, Nahles S, et al. Metal elements in tissue with dental peri-implantitis: a pilot study. Clin Oral Implants Res. 2016;27(9):1178-1186.
43. Fretwurst T, Nelson K, Tarnow DP, et al. Is metal particle release associated with peri-implant bone destruction? An emerging concept. J Dental Res. 2018;97(3):259-265.
44. Suarez-Lopez del Amo F, Rudek I, Wagner VP, et al. Titanium activates the DNA damage response pathway in oral epithelial cells: a pilot study. Int J Oral Maxillofac Implants. 2017;32(6):1413-1420.