You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repetitive episodes of upper airway obstruction that occur during sleep. During an OSA episode, a person stops breathing for 10 seconds or more as a result of airway collapsibility, resulting in the reduction of blood oxygen saturation that ends with sleep arousals.1 OSA is a common disease that is largely underdiagnosed and untreated with significant implications for cardiovascular disease,2 mortality,3 and economic impact.4 Population-based epidemiologic studies have estimated the prevalence and severity spectrum of undiagnosed OSA, and have found that even mild OSA is associated with significant morbidity.5
Diagnosis
The gold standard diagnostic test for OSA is overnight polysomnography (sleep study). This involves recording multiple physiologic signals during sleep, including an electroencephalograph (EEG), electrooculogram (EOG), electromyogram (EMG), oronasal airflow, oxyhemoglobin saturation, respiratory effort measurement, and other physiologic measurements.6 The updated manual for the scoring of sleep and associated events defines an apneic event as a reduction of airflow by 90% or more for 10 seconds with persistent effort to breathe; a hypopnea event is characterized by a 30% or greater reduction in airflow with either a 3% arterial oxygen desaturation (the previous threshold was a 4% arterial oxygen desaturation), an arousal, or both. An associated event known as respiratory effort–related arousal (RERA) is a sequence of breaths lasting at least 10 seconds characterized by increasing respiratory effort leading to an arousal from sleep.7
Severity
Apneas, hypopneas, and RERAs are used to determine the severity of OSA. Severity is measured by two indices: the first, known as the apnea-hypopnea index (AHI), measures the sum of apneas and hypopneas per hour of sleep. The second measure, known as the respiratory disturbance index (RDI), measures the sum of apneas, hypopneas, and RERAs per hour of sleep. Each index (AHI and RDI) will establish severity based on the average number of episodes per hour of sleep. An index of less than five is normal; an index of five to 15 indicates mild OSA; an index of 15 to 30 indicates moderate OSA; an index of greater than 30 indicates severe OSA.1
Common Symptoms
Common symptoms associated with OSA are habitual loud snoring and nocturnal breathing pauses, gastroesophageal reflux (GERD), and excessive daytime sleepiness.8
Snoring
Almost all sleep apnea patients snore and their snoring can be extremely loud. A characteristic pattern in sleep apnea is that of loud snoring or brief gasps that alternate with episodes of silence (breathing pauses) witnessed by the bed partner. The complaint of snoring precedes the complaint of daytime sleepiness, and the intensity of snoring increases with weight gain and bedtime alcohol intake.9
GERD
GERD is another frequently observed symptom among patients with sleep apnea. Increased breathing efforts during periods of apnea increase intra-abdominal pressures, while making the intrathoracic pressures more negative. Reflux results when an increased gradient between intra-abdominal and intrathoracic pressures favors the movement of gastric contents into the esophagus.10 While obtaining the medical history of dental patients with a complaint of GERD, it is important to screen for OSA.
Daytime Sleepiness
Daytime sleepiness is one of the most common clinical manifestations of OSA. Normal sleep is characterized by a pattern of four to five sleep cycles of non-REM (stage I to III) and REM sleep throughout the night. These cycles serve a restorative function. OSA is usually associated with disturbed sleep architecture. Upper airway obstruction during sleep induces central nervous activation, the so-called arousal, which is usually not noticed by the patient.11 The fragmentation of sleep tends to cause excessive daytime sleepiness.
Prevalence of OSA
Population-based epidemiologic studies have estimated a high prevalence of and wide severity spectrum for OSA. Data from the Wisconsin Sleep Cohort Study, a longitudinal study of the natural history of cardiopulmonary disorders of sleep, were used to estimate the prevalence of undiagnosed sleep-disordered breathing among adults.12 The estimated prevalence of sleep-disordered breathing, defined as an apnea-hypopnea score of 5 or higher, was 24% and 9% for men and women, respectively. The study showed that over a 4-year period, weight change was an important determinant of disease progression and regression.
The 2005 National Sleep Foundation poll, which included 1,506 adults (775 women) with a mean age of 49 years, showed that one in four individuals of a representative sample of US adults are at risk to develop OSA.13 Those classified as high risk according to the Berlin questionnaire used in the poll were more likely to have a chronic illness and also more likely to report that sleep disturbances negatively affected their quality of life. In addition, the poll found that 57% of obese respondents could be classified as high risk for developing OSA.
Pathophysiology of Upper Airway Control
The upper airway, which extends from the posterior end of the nasal septum to the epiglottis, has relatively little bony support in humans. One key feature of sleep is the suppression of upper airway muscle activity; sleep-related decreases in this activity is thought to lead to pharyngeal narrowing or closure in patients with OSA.14
The pathophysiology leading to pharyngeal collapse involves a combination of anatomic and physiologic influences.15 The pharyngeal airway is a collapsible tube that depends on transmural pressure across the pharyngeal wall for its patency. The forces necessary to maintain an adequate upper pharyngeal patency or collapse the upper airway are known as intraluminal and extraluminal pressures, respectively. Intraluminal pressure is negative pressure generated by the diaphragm during inspiration and extraluminal pressure results from gravitational force acting on the tissues and bony structures surrounding the airway.16
Intraluminal Pressure
Intraluminal pressure reduces airway patency. During each inspiration, the negative pressure generated by the diaphragm diminishes airway size depending on the muscle function of the airway walls and opposing dilating forces.
Schwartz and colleagues used critical closing pressure (Pcrit) to describe the airway pressure required to collapse the pharyngeal airway.17 To examine the relationship between Pcrit and the development of upper airway occlusion, they considered the relationship between nasal pressure and maximal inspiratory airflow. At varying levels of subatmospheric pressure applied to a nasal mask during sleep, maximal inspiratory airflow decreased in proportion to the level of nasal pressure. When nasal pressure fell below a Pcrit, subjects demonstrated upper airway occlusion terminated by arousals from sleep. Critical closing pressure is not a product of hypopharyngeal pressure but rather the pressure generated by respiratory muscles that can reduce upper airway size, but generally without collapsing the airway.
Subatmospheric intraluminal pressure is the most widely accepted theory of upper airway obstruction during sleep. According to the balance of forces theory, upper airway obstruction occurs when the collapsing intraluminal pressure generated by the thoracic muscles exceeds the dilating forces generated by upper airway dilator muscles. Remmers and colleagues presented a landmark study showing that obstructive apnea occurs when genioglossus muscle activity decreases and negative pressure continues to be generated.15
Extraluminal Pressure
Extraluminal pressure also increases transmural pressure and promotes obstruction of the upper airway. Examples of collapsing extraluminal pressure include passive gravitational forces generated by the craniofacial structures or adipose tissue surrounding the upper airway.18 The occurrence of complete upper airway obstruction in the absence of negative intraluminal pressure suggests that the upper airway collapsed due to extrinsic pressure.
Isono and colleagues19 compared the mechanism of the pharynx of anesthetized, paralyzed normal subjects and patients with OSA. In normal subjects, the pharynx was maintained at atmospheric intraluminal pressure and required negative intraluminal pressure for closure. Patients with OSA demonstrated positive closing pressure, meaning the pharynx was closed at atmospheric intraluminal pressure; therefore, the surrounding extraluminal pressure might induce upper airway obstruction during sleep. The extraluminal forces explain how external pressure and gravity influence the upper airway patency.
There are several anatomic and physiologic factors that promote the occurrence of episodes of OSA. In addition to the above, there are several local anatomic abnormalities such as the decrease of activation of the autonomous nervous system, obesity generating an increased pressure from fat tissue, and central nervous system depression from alcohol or drug consumption.20
Medical Consequences of Untreated OSA
Cardiovascular Disease
Growing evidence suggests an independent association between OSA and cardiovascular disease. Peker and colleagues21 explored the incidence of cardiovascular disease using a consecutive sleep clinic cohort of 182 middle-aged men (mean age 46.8 years) with and without OSA. At baseline, all subjects were free of hypertension or other cardiovascular disease, pulmonary disease, diabetes mellitus, psychiatric disorders, alcohol dependency, and malignancy. In addition to examining the effectiveness of OSA treatment, the study noted age, body mass index (BMI), systolic and diastolic blood pressure at baseline, and smoking habits. The authors concluded that the risk of developing cardiovascular disease is increased in middle-aged OSA subjects independent of age, BMI, smoking status, and systolic and diastolic blood pressure. The body of evidence linking OSA and cardiovascular disease continues to grow, as recent studies show an increased incidence of coronary events, heart failure, stroke, and cardiac mortality.22
Diabetes
Sleep-disordered breathing is commonly found in patients with type 2 diabetes. Using the Berlin questionnaire, West and colleagues23 surveyed men with type 2 diabetes from local hospitals and selected primary care databases about snoring, apneas, and daytime sleepiness to estimate the prevalence of OSA in this group. Selected respondents were monitored with pulse oximetry to establish whether they had OSA. Of the 1,682 men who received questionnaires, 56% replied; 57% of respondents scored as high risk and 39% as low risk for OSA. Results were verified by detailed sleep studies. BMI and diabetes were significant independent predictors of OSA. The author concluded that OSA is highly prevalent in men with type 2 diabetes, but most are undiagnosed and diabetes itself may be a significant independent contributor to the risk of OSA. There has long been a recognized association between type 2 diabetes and OSA, and there is emerging evidence that this relationship is likely to be at least partially independent of adiposity. A report from the International Diabetes Federation Task Force on Epidemiology and Prevention of Diabetes24 strongly recommends that health professionals working with patients with type 2 diabetes or sleep-disordered breathing adopt clinical practices to ensure that a patient presenting with one condition is considered for the other. The evidence of an association between diabetes and OSA emphasizes the need for increased awareness and screening for OSA in patients reporting the presence of diabetes.
Erectile Dysfunction
Erectile dysfunction (ED) was noted as a symptom of OSA as early as 1977.25-27 ED is age-related, especially common in men between 50 and 75 years old, and in men with chronic diseases like diabetes mellitus.28 Guilleminault and colleagues were the first to report ED or low libido in men with OSA.25 They estimated that 48% of men with OSA have these problems. Schmidt and colleagues29 were the first to identify OSA in patients presenting with ED. Pressman and colleagues confirmed these findings.26 diagnosing sleep apnea in 29% of patients presenting with impotence. Goncalves and coinvestigators30 evaluated the effect of 1 month of continuous positive airway pressure (CPAP) in a subgroup of OSA patients with ED and compared this subgroup with age- and BMI-matched OSA patients without ED. Their study concluded that ED in OSA is related to nocturnal hypoxemia, and about 75% of OSA patients with ED treated with nasal CPAP showed remission of their ED at 1-month follow-up, resulting in significant improvement in quality of life.
Mortality
Sleep apnea poses an independent risk for death, particularly from cardiovascular disease. Campos-Rodriguez and colleagues31 performed a historical cohort study of OSA patients (mean age 55 years and 80% male) treated with positive air pressure (PAP) therapy for approximately 5 years. To assess whether mortality was influenced by PAP therapy compliance, patients were assigned to one of the following categories of PAP therapy used: less than 1 hour per day, 1 to 6 hours per day, or more than 6 hours per day. By the end of the follow-up period (mean duration 48.5 months), 46 patients died. The 5-year cumulative survival rates were significantly lower in patients who did not use PAP (compliance <1 hour) than in those who used the PAP device for more than 6 hours per day. The study concluded that mortality rates in OSA patients who did not receive PAP therapy were higher compared with those who were treated with PAP and moderately or highly compliant with therapy. Patients died mainly from cardiovascular disease.
The Practice of Dental Sleep Medicine
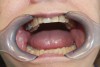
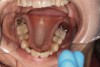
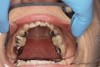
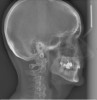
Dental sleep medicine (DSM) is a field in dentistry dedicated to the use and research of oral appliance therapy for the management of snoring and OSA. Oral appliance therapy is a noninvasive alternative to PAP therapy. Oral appliances fabricated by dentists with training in dental sleep medicine are commonly used to reposition the lower jaw forward to increase upper airway patency. Dental professionals have been recognized as being part of the multidisciplinary therapeutic team for the management of OSA because of their prime position of constant examination of the oropharyngeal areas. There are several oropharyngeal features contributing to the development of a narrow upper airway. These features are easily identifiable during a routine dental appointment, such as a large and scalloped tongue (Figure 1), narrow maxilla (Figure 2), small mandible (Figure 3), and retrognathic mandible (Figure 4). Combining clinical history intake, oropharyngeal examination, and the use of validated screening questionnaires, such as the STOP-Bang, dentists can screen for signs and symptoms of OSA and make recommendations for further diagnostic testing.
The leading professional association promoting the use and research of oral appliance therapy is the American Academy of Dental Sleep Medicine (AADSM), which offers educational courses throughout the year in addition to an annual meeting. The teaching of DSM has also been successfully incorporated into the pre- and postgraduate programs in some dental schools and it is expected that this will continue to expand into other universities across the country. The new clinical practice guideline for oral appliance therapy recommends that all dentists involved in this field possess additional training in DSM provided by DSM-focused nonprofit organizations or accredited dental schools. Pediatric dentists and orthodontists also have an important role in the screening and early detection of children with sleep-disordered breathing. Identification of altered craniofacial growth patterns, signs, symptoms, risk factors, and comorbidities of pediatric sleep-disordered breathing in daily practice may assist early intervention, preventing the need for management of a disease later in life.
Conclusion
As professionals committed to comprehensive care, dentists are concerned about any medical condition(s) that could compromise the health of their patients and their patients’ dental experiences. As the DSM field continues to grow, it is important that dentists involved in this field understand the importance of adhering to ethical and professional guidelines and develop clinical protocols in their practices to maintain an interdisciplinary collaboration with sleep physicians, cardiologists, and otolaryngologists, among other medical specialties.
Author Information
Leopoldo P. Correa, BDS, MS, is an associate professor and program director of the dental sleep medicine fellowship program at Tufts University School of Dental Medicine in Boston. He received his dental degree from the University of Veracruz in Mexico. He continued his education at Tufts University School of Dental Medicine, where he received his clinical training in temporomandibular disorders (TMDs) and obtained a Master of Science degree. He assisted with the incorporation of the dental sleep clinic into the existing TMD center at Tufts, where he has treated obstructive sleep apnea and TMD patients for more than 10 years. Dr. Correa advocated for the teaching of dental sleep medicine into the pre- and postgraduate programs at Tufts. He is a diplomate of the American Board of Dental Sleep Medicine and has participated as a speaker in numerous seminars nationally and internationally.
Disclosure
Leopoldo P. Correa, BDS, MS, has no relevant conflicts of interest to disclose.
References
1. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667-689.
2. Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA.2003;290(14):1906-1914.
3. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31 (8):1071-1078.
4. AlGhanim N, Comondore VR, Fleetham J, et al. The economic impact of obstructive sleep apnea. Lung. 2008;186(1):7-12.
5. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9): 1217-1239.
6. Jafari B, Mohsenin V. Polysomnography. Clin Chest Med. 2010;31(2):287-297.
7. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep. 2012;8(5): 597-619.
8. Wenner JB, Cheema R, Ayas NT. Clinical manifestations and consequences of obstructive sleep apnea. J Cardiopulm Rehabil Prev. 2009;29(2): 76-83.
9. Kales A, Cadieux RJ, Bixler EO, et al. Severe obstructive sleep apnea—I: Onset, clinical course, and characteristics. J Chronic Dis. 1985;38(5):419-425.
10. Hawrylkiewicz I, Plywaczewski R, Dziedzic D, et al. [Gastroesophageal reflux disease (GERD) in patients with obstructive sleep apnoea syndrome (OSAS)]. Pneumonol Alergol Pol. 2006;74 (4):361-364.
11. Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181(9):997-1002.
12. Young T, Palta M, Dempsey J. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235..
13. Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population. Chest. 2006;130(3):780-786.
14. Eckert DJ, McEvoy RD, George KE, et al. Genioglossus reflex inhibition to upperairway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581(Pt 3): 1193-1205.
15. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931-938.
16. Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26(7):851-856.
17. Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64(2):535-542.
18. Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148(2):462-466.
19. Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82 (4):1319-1326.
20. Guilleminault C. [Sleep apnea syndrome]. Presse Med. 1984;13(7):433-436.
21. Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middleaged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159-165.
22. Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26(6):541-547.
23. West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61(11):945-950.
24. Shaw JE, Punjabi NM, Wilding JP, et al. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract.2008;81(1):2-12.
25. Guilleminault C, Eldridge FL, Tilkian A, et al. Sleep apnea syndrome due to upper airway obstruction: a review of 25 cases. Arch Intern Med. 1977;137 (3):296-300.
26. Pressman MR, DiPhillipo MA, Kendrick JI, et al. Problems in the interpretation of nocturnal penile tumescence studies: disruption of sleep by occult sleep disorders. J Urol. 1986;136(3):595-598.
27. Fanfulla F, Malaguti S, Montagna T, et al. Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep. 2000;23 (6):775-781.
28. Shiri R, Koskimaki J, Hakama M, et al. Effect of chronic diseases on incidence of erectile dysfunction. Urology. 2003;62(6):1097-1102.
29. Schmidt HS, Wise HA 2nd. Significance of impaired penile tumescence and associated polysomnographic abnormalities in the impotent patient. J Urol. 1981;126(3):348-352.
30. Goncalves MA, Guilleminault C, Ramos E, et al. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005;6(4):333-339.
31. Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624-633.
About the Author
Leopoldo P. Correa, BDS, MS
Associate Professor
Program Director, Dental Sleep Medicine Fellowship
Craniofacial Pain Center
Department of Diagnostic Sciences
Tufts University School of Dental Medicine
Boston, Massachusetts