You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Stem-cell research has made significant progress regenerating various types of dental hard and soft tissues. However, growing a complete tooth with the specific intricate properties and interaction of these tissues remains a great challenge. In the meantime, conserving and restoring missing tooth structure offers the best option to maintain and retain natural teeth as long as possible.
The first part of this article relates to restoring missing or lost tooth structures. The entire armamentarium of restorative and prosthodontic treatment options—from fissure sealants to complete dentures—is available for any given patient situation. Severe tooth structure loss may require complete reconstruction with a full-coverage restoration, ie, a crown. The full-coverage crown, however, should always be the last treatment option because it is the most invasive and traumatic restorative procedure. This is due to the fact that a retentive preparation design to accommodate it has to be employed.
There are several restorative treatment options for situations of light to moderate tooth structure loss. Most of these rely on techniques that bond restorative materials to the remaining tooth structure. These are referred to as “adhesive dentistry” and range from direct composite to bonded indirect restorations. These restorations do not require excessive and damaging tooth structure removal and ultimately allow patients to retain their natural teeth longer, delaying the destructive cascade of crowns, endodontic treatment, and, ultimately, tooth replacement.
Adhesive Dentistry
Adhesive dentistry relates to bonding direct (ie, composite resins) and indirect (ie, ceramics) restorations to the teeth. Understanding how adhesive dentistry, or “bonding,” works is important to properly apply these techniques and provide long-term clinical success, as the various tooth structures and dental materials require specific bonding protocols. For enamel and dentin, the etch-and-rinse (total-etch) and self-etch adhesive concepts have advantages and disadvantages in different clinical situations. Self-etch adhesives, particularly two-step systems, provide excellent bond strength to dentin. They are, therefore, recommended for cavities that are predominantly in dentin. Etch-and-rinse systems, which typically include pretreatment with phosphoric acid, are preferred for indirect restorations and cavities that are mostly in enamel.1
The first step in any treatment is a proper diagnosis and a treatment plan based on the needs and individual situation of the patient. During the treatment phase, the preparation should be geared toward the preservation of tooth structure, especially enamel, which is the preferred substrate for bonding. For indirect restorations, such as ceramics, however, preparation designs must balance the need to provide sufficient space for the restoration with the goal to preserve as much tooth structure as possible. Contamination with moisture, saliva, and blood is the enemy of adhesive dentistry. Therefore, proper isolation—for example, with a rubber dam—is essential during bonding procedures.
Direct composite restorations offer minimally invasive treatment options in small to moderate cavities. More extensive tooth decay may require indirect restorations, such as laminate veneers and inlays/onlays, due to the polymerization shrinkage and limited inherent physical properties of composite resin materials.
Composites and ceramics are popular indirect materials. To achieve adequate bond strength to indirect composites, the preferred treatment is a combination of air-particle abrasion and the application of a silane coupling agent.2 The two major categories of all-ceramic materials are silica-based (ie, feldspathic, leucite-reinforced, and lithium disilicate) and non-silica–based (ie, zirconia, alumina) high-strength ceramics.
Silica-based ceramics, which are often used for laminate veneers, are preferably etched with hydrofluoric acid, followed by the application of a silane coupling agent.3,4 Hydrofluoric acid selectively dissolves the glass or weak crystalline components of the ceramic and produces a porous surface of increased wettability. The silane coupling agent on the etched ceramic surface increases the chemical adhesion between the ceramic and resin materials by coupling the silica (silicon oxides) in glassy matrix ceramics to the organic matrix of resin materials by means of siloxane bonds.4
The high strength of alumina and zirconia ceramics allows for cementation of full-coverage restorations with conventional cements. They can also be used for bonded restorations, such as resin-bonded bridges. However, the bonding methods used for silica-based ceramics are not efficient for high-strength ceramics.5 The preferred surface treatment methods include air-particle abrasion with aluminum oxide and the application of a special ceramic primer containing a special acidic adhesive monomer that has the ability to chemically bond to metal oxides. Silica coating followed by silanization or chemical activation has also revealed high bond strengths.5
Case 1
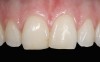
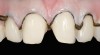
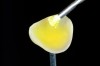
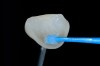
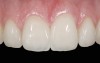
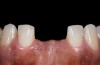
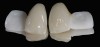
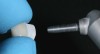
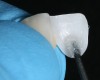
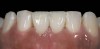
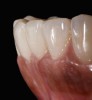
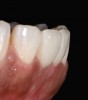
The clinical application of this protocol is illustrated in a patient situation where laminate veneers were placed on four maxillary incisors to replace lost tooth structure and restore function and esthetics. The failing restorations were removed, followed by a conservative veneer preparation (Figure 1 and Figure 2). The bonding surfaces of the feldspathic porcelain veneers were acid-etched with hydrofluoric acid for 2 minutes (Figure 3) and thoroughly rinsed. Then a silane coupling agent was applied (Figure 4). Figure 5 demonstrates the situation after bonding the veneers to the teeth with a composite resin luting agent.
Case 2
High-strength ceramic materials can be used in select cases to fabricate resin-bonded bridges to replace missing teeth as an alternative to removable prostheses or more invasive procedures such as conventional bridges or dental implants. The two missing lower central incisors (Figure 6) were replaced with two zirconia-based resin-bonded bridges (Figure 7). The single-retainer wing design has shown more than 94% clinical success after 10 years, which is significantly higher than the conventional two-retainer design (67.3% success).6 Proper bonding, however, is key for success and employs an air-particle abrasion step (Figure 8) followed by the application of a special ceramic primer (Figure 9). Figure 10 through Figure 12 demonstrate the clinical outcome.
For thin porcelain laminate veneers, it is preferred to use a light-cure composite resin luting agent. Dual-cure or self-cure composite resins are indicated for indirect porcelain restorations that are more than 2 mm thick or more opaque (eg, zirconia). Whenever light-cure or dual-cure composite resins are used, it is recommended to light-cure these restorations sufficiently (at least 60 seconds from every side).
More recently, self-adhesive resin cements have become popular because they provide some bond strength to teeth and restorative materials without numerous pretreatment steps. They are, therefore, easy to use while offering some advantages over conventional cements. However, they do not provide sufficient bond strengths for indirect adhesive restorations such as laminate veneers and bonded bridges. They are the cements of choice for many practitioners for tooth- and implant-supported crowns and bridges.
Until tooth regeneration becomes a realistic option in the clinical world, dental restorations are the only option to re-create function and esthetics in the event of tooth structure loss that will allow patients to retain their teeth for as long as possible. Adhesive dentistry allows for minimally invasive restorations, but requires a fundamental understanding of tooth and material properties for long-term durable bond strengths.
Regenerating Bone and Periodontal Tissues
Today, clinicians use recombinant growth factors with various bioabsorbable or absorbable matrices in both periodontal and bone-regenerative applications geared toward dental implants. Practitioners should consider the following regenerative factors in the immediate implant placement decision: combination therapy using biologics, growth factors, types of bone grafts, and some forms of membranes.
Such combination therapy enables the practitioner to avoid removing healthy tissue from the attachment apparatus of the tooth being treated as well as the adjacent one, thus providing a scaffold for the bone to grow. These bioactive molecules, or growth factors, are delivered and provide space maintenance for the scaffold. They offer the means for achieving the ultimate objective: to regenerate the lost tissues to improve esthetics both for patients and restorative collagen.
The mechanism of action for recombinant human platelet-derived human growth factor-BB (rhPDGF-BB) is as follows7: This growth factor is released from the carrier, and then binds to tyrosine kinase receptors on the receptor’s cell. As a potent chemotactic agent, it pulls stem cells to the desired site of regeneration; and as a very powerful mitogen, it causes cell division and population to increase at the desired site. The primary matrix, which forms in approximately 6 months, becomes a mature periodontal attachment apparatus.
Case 3
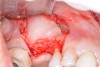
A patient in her 60s wanted to improve her smile but declined orthodontic treatment in favor of porcelain veneers (Figure 13). However, addressing her 8-mm pocket was the first step. The practitioner created biologically clean root surfaces using ultrasonic open-flap debridement, finishing burs, and manual tool instrumentation. Next, a mineralized freeze-dried bone allograft saturated in platelet-derived growth factor (Figure 14) using chemotaxis pulled the stem cells capable of regeneration into the site where repopulation was needed. Prior to closure, a resorbable membrane, polylactic acid-guided tissue regeneration—known for periodontal regeneration—was used (Figure 15). At 10 weeks, adequate healing had occurred. The patient at 6 and 10 weeks showed continued healing. Although some postoperative recession was evident, probing depths were less than 2 mm, and the patient was referred back to the restorative dentist for restorative therapy. As of this writing, she has not lost any teeth and is periodontally stable (Figure 16).
Case 4
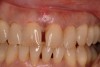
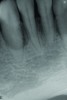
In a case with an intrabony defect, such as with the patient in Figure 17, a periodontist might suggest sacrificing supporting bone to remove the osseous defect. Doing this might lead to issues related to tooth mobility, esthetics, caries, and hypersensitivity.
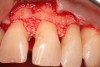
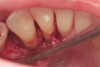
The patient had been referred for an implant consultation for the mandibular right canine. She had very deep probing depths on the lingual. When the flap was raised, a significant amount of subgingival calculus was seen as a local risk factor (Figure 18). However, she had no significant pathology to remove from the situation. Debridement was performed the same as in Case 3 with the rotary ultrasonic and manual instrumentation.
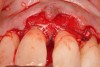
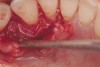
The defect was obturated with mineralized freeze-dried bone and hydrated with platelet-derived growth factor. As with any growth factor, when implementing it clinically, all growth factors require a carrier to bring that growth factor to the desired site, which do not elicit an inflammatory response. In this situation, it is the mineralized freeze-dried bone allograft. This combination was demonstrated by Rosen et al8 to be efficacious in treating intrabony defects. A biologic approach was taken both in terms of the graft and membrane (Figure 19). Primary closure was achieved.
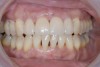
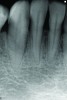
At the 13-month postoperative visit, the patient who had initially been referred for extraction and an implant still has the tooth with minimal probing depths and no mobility (Figure 20). She reported that she is happy that she did not have to have her canine extracted.
Case 5
Instead of performing root-coverage surgery on a patient referred for a large amount of gingival recession, the surgeon used an acellular dermal allograft, hydrating it with the platelet-derived growth factor.9 In the literature, combining rhPDGF-BB with autogenous connective tissue grafts for root coverage has been demonstrated.10 In this case, due to the large number of teeth requiring treatment, a dermal allograft was chosen to minimize the overall number of surgeries. Another approach, comparable with subepithelial connective tissue grafts, is to use rhPDGF-BB with a carrier and collagen membrane, as demonstrated by McGuire and Scheyer.11
It involved raising a flap after root planing and conditioning, and suturing this allograft material around all eight teeth being treatment planned for root coverage. Periosteal release and a coronally advanced tissue flap were provided for the entire growth factor-enriched allograft. Evaluations were performed at 10 days, 3 weeks, and 6 months. At 18 months, the situation was stable, with a small amount of residual recession with the posterior.
Case 6
In a case similar to Case 1, a maintenance patient in her late 60s received open debridement. The carrier that is marketed with this rhPDGF material is a beta-tricalcium phosphate (beta-TCP) alloplast. Although this type of material is not known to be successful in providing regeneration, Ridgway et al12 concluded that regeneration was mainly caused by the growth factor, and the alloplast present may have actually prevented more bone formation and regeneration. Another property of rhPDGF is that it is a mitogen, which causes these stem cells to divide, so more regenerate into cells such as osteoblasts. After the defect is debrided, the chemotactic property draws these cells to the site, and then the growth factor causes these cells to divide.
The mineral allograft hydrated with growth factor was then placed into the defect. Bone was placed where growth was desired. Amnion/chorion material was used, and closure was completed. The 1-year result was a small amount of postoperative recession and nearly complete resolution of vertical defect, with no tooth loss.
Bone Morphogenetic Proteins
To grow new tissue, some form of active cells is needed for the process, and those cells need to be stimulated through a signal.13 Some kind of matrix is necessary to allow this regeneration to occur. With regeneration using bone morphogenetic protein-2 (BMP-2), the BMP-2 is the signal, the growth factor. One of the properties of BMP-2 is a chemotactic growth factor that brings cells into the desired site of regeneration.
The growth factors of BMP-2 are a part of the TGF-beta superfamily of growth factors. They activate a transcription factor in the cell to carry a message to the nucleus and gene expression will occur, causing this stem cell to become an osteoblast and form new bone. Because it is chemotactic, it pulls undifferentiated stem cells to the desired site of regeneration, and because it is a morphogen, it turns these stem cells into bone. A robust angiogenic response occurs, because BMP-2 will bind to cells and cause cells to produce vascular endothelial growth factor. The result is new bone formation. What is desired is the matrix degradation without an inflammatory response, and modeling and remodeling of new bone. In short, BMP-2 is a morphogen and causes these stem cells, once contacted by BMP-2 , to become strong bone-forming osteoblasts. That is what truly differentiates BMP-2 from other available growth factors.
Case 7
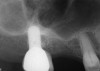
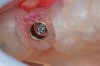
A patient who had received treatment 10 years ago for implants in the mandibular arch said her general dentist wanted to place an implant in site No. 3, using a hammer. Although that clinician believed the site had sufficient bone based on a periapical x-ray findings (Figure 21), the findings from a cross-sectional computed tomography scan helped determine that she had less than 3 mm of bone in the ridge (Figure 22). Froum et al14 and Tarnow et al15 both demonstrated the efficacy of combining rhBMP-2/ACS with mineralized bone allograft in sinus graft procedures. Marx et al16 demonstrated the combination of this growth factor with mineralized allograft and PRP to reconstruct severe maxillary defects. Given the 4 mm of minimal requirement for simultaneous implant placement, the author performed a lateral window sinus graft. An absorbable collagen sponge hydrated with rhBMP-2 was combined with mineralized bone allograft and used to obturate the site after reflection of the Schneiderian membrane along the medial sinus wall. A portion of the rhBMP-2/ACS was adapted over the grafted window osteotomy (Figure 23). After healing for approximately 5 months, the patient received a computer-guided implant surgery (Figure 24). The implant was restored with a screw-retained crown after a healing period of approximately 3 months.
Conclusion
Practitioners must grapple with myriad approaches to partial or total edentulism. Today, it is often possible to save a tooth rather than replace it. Regenerative therapy already improves outcomes in patients with lost periodontal attachment, in mucogingival applications, with sinus and ridge augmentations, and with simultaneous implant placement.
Restoring comfort, function, and esthetics to a patient in her 40s who has already had a failed bridge could mean providing a long-span tooth-borne bridge or a partial denture—both unlikely to provide a lasting or satisfactory solution. A better approach would be to return the case to a fixed situation that is healthy and stable with hard and soft tissues around implants. A patient with congenitally missing lateral incisors, who is likely to also be missing the alveolar ridge in that site, can have the defect repaired using regenerative therapies prior to implant placement. Combining these growth factors with allografts and resorbable scaffolds is opening the door to great possibilities.
About the Authors
Barry P. Levin, DMD
Clinical Associate Professor
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
Private Practice limited to Periodontics and Dental Implant Surgery
Elkins Park, Pennsylvania
Markus B. Blatz, DMD, PhD
Professor of Restorative Dentistry, Chairman of the Department of Preventive and Restorative Sciences
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
President
International Academy for Adhesive Dentistry
Disclosure
The authors disclose that they have no financial relationships to any manufacturers of products mentioned in this article.
References
1. Ozer F, Blatz MB. Self-etch and etch-and-rinse adhesive systems in clinical dentistry. Compend Contin Educ Dent. 2013;34(1):12-18.
2. Özcan M, Pekkan G. Effect of different adhesion strategies on bond strength of resin composite to composite-dentin complex. Oper Dent. 2013;38(1):63-72.
3. Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89(3):268-274.
4. Blatz MB, Sadan A, Maltezos C, et al. In vitro durability of the resin bond to feldspathic ceramics. Am J Dent. 2004;17(3):169-172.
5. Blatz MB, Chiche G, Holst S, Sadan A. Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int. 2007;38(9):745-753.
6. Kern M, Sasse M. Ten-year-survival of anterior all-ceramic resin-bonded fixed dental prostheses. J Adhes Dent. 2011;13(5):407-410.
7. Matsuda N, Lin WL, Kumar NM, et al. Mitogenic, chemotactic and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63(6):515-525.
8. Rosen PS, Toscano N, Holzclaw D, Reynolds MA. A retrospective consecutive case series using mineralized allograft combined with recombinant human platelet-derived growth factor BB to treat moderate to severe osseous lesions. Int J Periodontics Restorative Dent. 2011;31(4):335-342.
9. Levin BP. Stable root coverage with a dermal allograft enriched with rhPDGF-BB. Inside Dentistry. 2014;10(9):2-4.
10. Rubins RP, Tolmie P, Corsig KT, et al. Subepithelial connective tissue graft with growth factor for the treatment of maxillary gingival recession defects. Int J Periodontics Restorative Dent. 2013;33(1):43-50.
11. McGuire MK, Scheyer ET. Comparison of recombinant human platelet-derived growth factor-BB plus beta tricalcium phosphate and a collagen membrane to subepithelial connective tissue grafting for the treatment of recession defects: a case series. Int J Periodontics Restorative Dent. 2006;26(2):127-133.
12. Ridgway HK, Mellonig JT, Cochran DL. Human histologic and clinical evaluation of recombinant human platelet-derived growth factor and beta-tricalcium phosphate for the treatment of periodontal intraosseous defects. Int J Periodontics Restorative Dent. 2008;28(2):171-179.
13. Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. J Bone Joint Surg Am. 2002;84-A(6)1032-1044.
14. Froum SJ, Wallace S, Cho SC, et al. Histomorphometric comparison of different concentrations of recombinant human bone morphogenetic protein with allogeneic bone compared to the use of 100% mineralized cancellous bone allograft in maxillary sinus grafting. Int J Periodontics Restorative Dent. 2013;33(6):721-730.
15. Tarnow DP, Wallace SS, Froum SJ, et al. Maxillary sinus augmentation using recombinant bone morphogenetic protein-2/acellular collagen sponge in combination with a mineralized bone replacement graft: a report of three cases. Int J Periodontics Restorative Dent. 2010;30(2):139-149.
16. Marx RE, Armentano L, Olavarria A, Samaniego J. rhBMP-2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: an unsponsored randomized open-label clinical trial. Int J Oral Maxillofac Implants. 2013;28(5):e243-e251.