You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
During dental practice, a film of organic debris is accumulated on the surfaces of the instruments that must be removed before they are sterilized. It is composed of tissues, blood, and microorganisms from the patient. In the presence of organic matter, disinfectants demonstrate reduced activity. Likewise, the quantity of microorganisms on the surface of an instrument, or bioburden, affects the quality of disinfection. We define instrument cleaning as the removal of all traces of organic and inorganic matter from the surfaces of instruments. It is an essential step in the sterilization chain because the presence of these substances interferes with disinfection and can subsequently inactivate the results of sterilization.
In the 1860s, Joseph Lister recommended that surgical instruments be antiseptically cleaned to prevent the spread of infection, and since then, many cleaning and sterilization techniques have been developed. The primary factors involved in instrument cleaning are summarized in Sinner's Circle. In 1959, chemist Herbert Sinner identified the four components of effective cleaning—temperature, application time, mechanical action, and chemical action—which he depicted in a circle to demonstrate their relationship. The circle is not static. This means that if one of the components is modified, in order for cleaning to be successful, one or more of the other components will have to be modified to compensate. For example, if a material is sensitive to heat and must be cleaned at a reduced temperature, the mechanical action will have to applied for a longer period of time in the presence of the chemical agent.1Understanding the factors in Sinner's Circle helps us to design cleaning strategies for different types of instruments, and for most of these, the solvent is generally water.
Water varies in its composition based on the geology of the area. In locations where the water is harder, or has a higher mineral content, the repeated cleaning of instruments can result in the development of a calcareous film on their surfaces. Furthermore, the effectiveness of certain detergents is impacted by the presence of these dissolved mineral salts. Another problem related to hard water is corrosion caused by excess chlorides. To avoid these problems, the quality of the water used in cleaning must be standardized through the use of reverse osmosis systems or by using distilled water.
The first step in instrument processing is transportation to a sterilization room. To accomplish this transport, closed containers or cassettes should be used and appropriate personal protective equipment (PPE) should always be worn. The PPE, which consists of glasses or a face shield, a hat, a mask, a long gown, a waterproof apron, and thick gloves worn over latex gloves, should be used throughout the entire cleaning and sterilization process.1 In addition to reducing the risk of accidents, the use of container boxes, saves time, prevents the instruments from being damaged, and helps with organization, storage, and inventory. The time savings can be attributed to several factors, such as the ability to transport multiple instruments at once and single bagging, which facilitates the sterilization of many instruments at the same time without overloading the autoclave.2
In situations in which instruments are not going to be cleaned immediately, they should be soaked to prevent the organic material from drying out and making cleaning more difficult.3 For this soaking phase, the use of alcohol, salts, or aldehydes should be avoided. Ideally, an enzymatic detergent mixed in the proportions indicated by the manufacturer should be used to presoak instruments. However, to avoid corrosion, they should not be left to soak for too long. The temperature of the water also plays an important role. If very hot water is used, the organic material will "cook," making it difficult to remove.
Whether instruments are processed immediately after use or presoaked and then processed later, their cleaning can be performed either manually or mechanically (Figure 1). The method or methods used are largely determined by the needs of each instrument.
Manual Cleaning
For many reasons, manual cleaning is not the technique of choice for instrument processing. It should only be performed on instruments that cannot tolerate other types of mechanical cleaning, such as delicate microsurgical instruments or those that cannot be submerged.4 Because manual cleaning is conditioned to the subjectivity of the individual who performs it, it is not a reproducible process that can be validated. There is also a high risk that accidents will occur during the handling of the instruments. Lapses in concentration while manually processing the large volume of instruments that can accumulate can lead to accidental punctures. In addition, any droplets of water created by the process are contaminating not only the sterilization room but also the staff.5 Beyond contamination of the environment by the aerosols generated, manual cleaning also adds to the overall cost of processing as a result of both increased water consumption and the additional time required by staff.
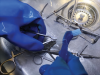
When possible, the manual cleaning of instruments should be performed underwater, to avoid splashing contaminated liquids (Figure 2). The instruments should be opened and disassembled throughout the process. To help staff remain as far away from contaminated instruments as possible and avoid accidents, cleaning should be caried out with long-handled brushes. Sharp instruments should be cleaned separately, and lumens should be cleaned using fine brushes that are specifically designed for them.
After cleaning, the instruments should be copiously rinsed, first with running water and then with distilled water (Figure 3). This helps to avoid the deposition of salts onto the instruments that can deteriorate them over time. Once properly rinsed, each instrument should be inspected to ensure that there are no remaining traces of organic matter and then meticulously dried.
Drying is a very important phase in which all residual moisture is removed from the instruments in order to prevent the deposition of mineral salts and the potential invalidation of the subsequent sterilization process. Autoclaves are programmed to evaporate the same amount of water that they introduce into the chamber; therefore, extra water resulting from improperly dried instruments can cause the envelopes to come out wet or stained and require reprocessing. According to sections 8 and 20 of UNE-EN 285:2016, a European standard addressing steam sterilization, the weight of the packaging after sterilization should not exceed a 1% increase.6 After cleaning and drying, hinged instruments and dental handpieces should be lubricated when indicated.
Mechanical Cleaning
To improve safety and achieve a more thorough cleaning, an automated process should be used for instruments that can tolerate it. Mechanical cleaning, using either an ultrasonic cleaner or a washer-disinfector, offers many advantages, including the following2:
• Reduced risk of puncture injuries
• Reduced risk of environmental contamination
• Reduced water consumption
• Greater cleaning efficiency
• Greater staff productivity
Ultrasonic Cleaners
Ultrasonic cleaning is one of the most widespread mechanical cleaning methods used in dentistry. The short periods of time between patients can make attempts at manual cleaning less than 100% effective. In addition, many instruments have nooks and crannies that are inaccessible to brushing. Ultrasonic cleaning units provide one of the most effective cleaning methods available because they have the advantage of being able to act on every surface of an instrument.7 To accomplish this, an ultrasonic cleaner generates high-frequency sound that is transferred to the liquid in the form of waves. These waves result in a pressure variation that causes cavitation of the liquid and the creation of microbubbles that implode and generate energy to release accumulated debris from instruments.
There is no consensus regarding the need to presoak instruments prior to ultrasonic cleaning. If an instrument is fairly free of debris, presoaking it in enzymatic detergents does not appear to provide a benefit. However, there is a clear benefit to presoaking an instrument if it has been heavily covered with blood during a procedure.8 Certain types of instruments cannot be put into ultrasonic baths. For example, handpiece rotors and fiberglass and diamond burs should not be ultrasonically cleaned because they can be damaged by the vibration. In addition, plastics and silicones should not be introduced into ultrasonic cleaners because they absorb energy and can reduce the efficacy of the cleaning process.
It is very important that the liquid added to ultrasonic cleaners is mixed in the proportions indicated and at the temperature recommended by the manufacturer. Once the bucket has been filled, the unit should be turned on to release any gas from the mixture prior to use. Instruments are placed into the basket such that they are completely submerged, avoid direct contact with the bucket, and do not overload the machine. Placement of the cover is important to prevent environmental contamination and reduce noise during operation. After the cleaning cycle is complete, the instruments should be rinsed with distilled water and thoroughly dried before bagging and sterilization.
Washer-Disinfectors
There is consensus among experts that washer-disinfectors are the cleaning method of choice for dental instruments.9-11 They offer a process of cleaning that is standardized, reproducible, capable of validation, and ultimately traceable. If all the steps of instrument processing (eg, washing, bagging, sterilization) can be integrated into a single program, traceability can be achieved. Performance requirements for washer-disinfectors are governed by the ISO 15883-1:2006 standard.12 The process used by washer-disinfectors is homogeneous in all of its phases, during which all parameters (eg, time, concentration, temperature) are controlled. When compared with other cleaning methods, it provides additional safety to the operators when handling and bagging the instruments, reduces environmental contamination, and increases productivity due to the shorter working time.
The best way to take advantage of the time savings is to use perforated containers. After using instruments, it is only necessary to close the container and place it in the washer-disinfector. If instruments are not going to be processed immediately, they can be soaked, but foaming agents should be avoided or the instruments should be rinsed very well afterward because the presence of excess foam from presoaking agents can lower the rinse pressure and interfere with the effectiveness of washer-disinfectors.
Loading a washer-disinfector is a process that requires staff training. First, it is necessary to disassemble or open up the instruments per the manufacturers' instructions to prepare them for cleaning. Next, the instruments are placed into the washer-disinfector in such a way that the nozzles are not obstructed and spray shadows are avoided, taking care to not to overload the machine. Washer-disinfectors have accessories that are designed to clean specific instruments and devices, such as handpiece rotors or chair suction hoses. Once the process is finished, it is important to remove instruments timely to avoid corrosion.
The thermal disinfection achieved by washer-disinfectors is regulated by the A0 value.12 This value represents the temperature-time relationship required to inactivate a given bacterial load. For example, an A0 value of 3,000 corresponds to 90ºC for 5 minutes or 80ºC for 50 minutes.
Washer-disinfectors perform cleaning in several stages that are modifiable. Although different programs can be designed according to the needs of the instruments to be washed, typical cycles include the following stages:
• Prewash. Cold water is used to remove gross organic debris.
• Cleaning. Instruments are washed with an appropriate detergent and water at a temperature between 40ºC and 60ºC. In places where there are many chlorides in the water that can promote corrosion, it is recommended to either neutralize it or use deionized water.
• Disinfection. Desalinated water at a temperature between 80ºC and 90ºC is applied for the duration specified by the A0 value.
• Drying. Instruments are perfectly dried and ready for packaging and sealing.
Considerations for Endodontic Files
Many authors, including as Bryson13, Nosouhian,14 and Van Eldik,15 have been concerned with studying the amount of organic debris that remains on endodontic files and the best methods of cleaning these instruments. Because endodontic files have very small nooks and crannies that fill with detritus after use, there are concerns regarding their potential to transmit prion diseases, such as Creutzfeldt-Jakob disease. Although there is no scientific evidence thus far of the existence of these prions in pulp tissues and there have been no instances of prion diseases transmitted through root canal treatment,16,17 the possibility should not be disregarded, and clinicians should be very demanding regarding the cleaning of endodontic files. One study by Van Eldik and colleagues compared three methods for cleaning endodontic files to a control group without cleaning. These methods included cleaning with a thermal washer-disinfector cycle, ultrasonic cleaning with the files placed inside a perforated container, and ultrasonic cleaning with the files loosely placed in the bath without a container. Placing the endodontic files loosely in the ultrasonic cleaner achieved the most efficient cleaning, resulting in an average of 98% of file surfaces being free of biologic debris.15
Cleaning Rotary Handpieces
Unfortunately, the external cleaning of handpieces without a complete sterilization process continues to be a common practice in dental offices.18 Laboratory studies, such as one conducted by Checchi and colleagues,19 have demonstrated contamination of the air and irrigation ducts. The remains of dental materials, traces of cellular tissues, blood, saliva, and microorganisms originating from the patient's mouth as well as the water pipes of the dental chair can accumulate inside turbines.20 During the use of a turbine, a back-suction phenomenon occurs in which the interior of the head becomes contaminated with fluids from the oral cavity. For this reason, rotary handpieces used in dentistry are classified as critical instruments and must undergo complete decontamination and sterilization.
The manufacturers' instructions of some handpieces recommend exterior cleaning, lubrication, and subsequent sterilization; however, several studies have shown that the oil layer can protect microorganisms during sterilization and prevent them from being completely eliminated.21 Although the sterilization of handpieces by autoclaving is essential, all autoclaves are not equally effective in accomplishing this. Only Class B autoclaves can ensure complete sterilization inside handpiece turbines. Class S autoclaves cannot do this.
There are also devices that clean a rotary handpiece by dragging out the dirt, disinfecting it, and lubricating it. This can help to extend the life of the instrument. Another option for cleaning rotary handpieces is the use of a washer-disinfector. These machines have accessories with specific nozzles for deep cleaning the interior of handpieces and disinfecting them. They should then be lubricated, bagged, and sterilized.
Conclusion
The proper cleaning of dental instruments before proceeding with their sterilization is essential. There are many different methods available, and clinicians need to choose the most appropriate ones according to their needs and the needs of the instruments. The automation of cleaning increases the efficiency of the process while making it reproducible, able to be validated, and traceable.22
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
About the Author
Gema Maeso Mena, DDS, PhD
Professor of Periodontics
Complutense University of Madrid
School of Dentistry
Madrid, Spain
Private Practice
Madrid, Spain
References
1. Offner D, Brisset L, Musset AM. Evaluation of the mechanical cleaning efficacy of dental handpieces. J Hosp Infect. 2019;103(1):e73-e80.
2. US Department of Labor, Occupational Safety and Health Administration. Bloodborne Pathogens Standard. Code of Federal Regulations, title 29 §1910.1030.
3. Kelsch NB. Team centred approach to instrument processing and infection control. Registered Dental Hygienist. 2016;36(12):39-50.
4. Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
5. Ofstead CL, Hopkins KM, Smart AG, Brewer MK. Droplet dispersal in decontamination areas of instrument reprocessing suites. Am J Infect Control. 2021. doi: https://doi.org/10.1016/j.ajic.2021.10.023.
6. Spanish Association for Standardization and Certification. UNE EN 285:2016 Sterilization - steam sterilizers - large sterilizers. European Standards website. https://www.en-standard.eu/une-en-285-2016-sterilization-steam-sterilizers-large-sterilizers/. Published July 27, 2016. Accessed December 23, 2021.
7. Cuny EJ. Cleaning instruments: a critical step to instrument reprocessing. Inside Dental Assisting. 2010;6(8):26-29.
8. Sanchez E, Macdonald G. Decontaminating dental instruments: testing the effectiveness of selected methods. J Am Dent Assoc. 1995;126(3):359-362, 364, 366.
9. Department of Health. Decontamination health technical memorandum 01-05: decontamination in primary care dental practices. https://www.england.nhs.uk/publication/decontamination-in-primary-care-dental-practices-htm-01-05/. Published March 26, 2013. Accessed December 23, 2021.
10. Haas B, Soto KJ, Day DS, et al. Mycobacterium hassiacum: a thermophilic Mycobacterium species to demonstrate thermal disinfection of medical devices. BMC Res Notes. 2020;13(1):140. doi:10.1186/s13104-020-04978-7.
11. Pankhurst CL, Coulter WA. Basic Guide to Infection Prevention and Control in Dentistry. 1st ed. Wiley-Blackwell; 2009.
12. International Organization for Standardization. ISO 15883-1:2006 Washer-disinfectors - part 1: general requirements, terms and definitions and tests. ISO website. https://www.iso.org/standard/41076.html. Published April 1, 2006. Accessed December 23, 2021.
13. Bryson LM, Fernandez Rivas D, Boutsioukis C. Cleaning of used rotary nickel-titanium files in an ultrasonic bath by locally intensified acoustic cavitation. Int Endod J. 2018;51(4):457-468.
14. Nosouhian S, Bajoghli F, Sabouhi M, et al. Efficacy of different techniques for removing debris from endodontic files prior to sterilization. J Int Oral Health. 2015;7(8):42-46.
15. Van Eldik DA, Zilm PS, Rogers AH, Marin PD. A SEM evaluation of debris removal from endodontic files after cleaning and steam sterilization procedures. Aust Dent J. 2004;49(3):128-135.
16. Head MW, Ritchie D, McLoughlin V, Ironside JW. Investigation of PrPres in dental tissues in variant CJD. Br Dent J. 2003;195(6):339-343.
17. World Health Organization. WHO tables on tissue infectivity distribution in transmissible spongiform encephalopathies. http://www.who.int/bloodproducts/tablestissueinfectivity.pdf . Updated October 30, 2010. Accessed December 23, 2021.
18. Smith GWG, Smith AJ, Creanor S, et al. Survey of the decontamination and maintenance of dental handpieces in general dental practice. Br Dent J. 2009;207(4):E7;160-161.
19. Checchi L, Montebugnoli L, Samaritani S. Contamination of the turbine air chamber: a risk of cross infection. J Clin Periodontol. 1998;25(8):607-611.
20. Spagnolo AM, Sartini M, Cristina ML. Microbial contamination of dental unit waterlines and potential risk of infection: a narrative review. Pathogens. 2020;9(8):651.
21. Edwardsson S, Svensatar G, Birkhed D. Steam sterilization of air turbine dental handpieces. Acta Odontol Scand. 1983;41(6):321-326.
22. Li Y, Lu Y, Cai R, et al. Monitoring the cleanliness of reusable surgical instruments in central sterile supply department by adenosine triphosphate method. J AOAC Int. 2021;qsab102. doi: 10.1093/jaoacint/qsab102.