You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Dental plaque is the community of microorganisms found on a tooth surface as a biofilm, embedded in a matrix of polymers of host and bacterial origin.1,2 Plaque is natural and contributes (like the resident microflora of all other sites in the body) to the normal development of the physiology and defenses of the host.3-5 Plaque bacteria generally have a harmonious relationship with the host; they use endogenous nutrients (eg, salivary proteins and glycoproteins, such as mucin) for their growth, from which there is little acid production, and their presence helps exclude exogenous microorganisms (colonization resistance).
Formation Of Dental Plaque Biofilms
Dental plaque forms via an ordered sequence of events, resulting in a structurally and functionally organized species rich microbial biofilm.1,2 There are distinct stages in plaque formation:
- Acquired pellicle formation when molecules derived mainly from saliva are adsorbed onto the tooth surface.
- Reversible adhesion, which involves weak, long-range, physicochemical interactions between the microbial cell surface and the acquired pellicle.
- Irreversible adhesion, which involves interactions between specific molecules on the microbial cell surface (adhesins) and complementary receptors present in the acquired pellicle; these interactions are stronger and operate over a relatively short distance.
- Coadhesion, in which secondary colonizers adhere via cell surface adhesins to receptors on already attached bacteria6, leading to an increase in microbial diversity within the developing biofilm. Many of the secondary colonizers have fastidious growth requirements.
- The attached cells multiply, leading to an increase in biomass and synthesis of exopolymers to form the biofilm matrix. Numerous biochemical (eg, metabolic cooperation to catabolize mucins for growth) and molecular (cell-to-cell signaling to coordinate gene expression) interactions take place among the constituent species. The metabolism of these microorganisms leads to the development of gradients within the biofilm; for example, aerobic and facultatively anaerobic bacteria consume oxygen and produce carbon dioxide and hydrogen, making conditions suitable for the growth of obligate anaerobes. These processes lead to the establishment of a mature biofilm. Of clinical relevance is that biofilms such as dental plaque are less susceptible to antimicrobial agents when compared with the same cells growing in conventional liquid culture (planktonic cells). The structure of the plaque biofilm can restrict the penetration of antimicrobial agents because of the presence of charged exopolymers in the matrix, while bacteria growing on a surface divide only slowly and display novel properties, one consequence of which is a reduced sensitivity to inhibitors.7 Horizontal transfer of resistance genes is highly efficient in biofilms.
The microbial composition of mature plaque biofilms is diverse. Many of the microorganisms are fastidious and difficult to grow in the laboratory; conventional culture techniques introduce a bias in that they favor the growth of those species that grow well (and quickly) on standard bacteriologic agar plates. Modern molecular ecology approaches, in which 16S rRNA genes are amplified directly from plaque samples, have revolutionized our understanding of the oral microflora diversity.
Organisms no longer have to be grown; their identity can be deduced from the similarity of the amplified DNA to sequences stored in large databases. Within the rRNA gene, some stretches of DNA sequence are conserved while other areas are highly variable and reflect evolutionary divergence. These genes are short enough for rapid sequencing in well-equipped laboratories (using automated DNA sequencing equipment) but long enough to provide valuable discriminatory information to demonstrate similarities and differences among strains. These approaches have led to the identification of approximately 700 microbial taxa when data from numerous epidemiologic studies are combined, of which approximately 50% cannot be cultured in vitro. About 12 to 27 species predominate in individual plaque samples from healthy sites.8 The microflora varies in composition at different surfaces on teeth, with each surface having a characteristic composition. Fissures are colonized mainly by gram-positive bacteria, especially streptococci, and the microflora of this site is influenced by saliva. The gingival crevice has mainly anaerobic organisms, many of which are gram-negative, and gingival crevicular fluid is the major nutritional influence. This illustrates an important concept: the numbers and types of bacteria that are able to colonize successfully reflect the local biologic and physical conditions of the site. Therefore, a change in a key environmental parameter could lead to an alteration in the competitiveness of particular species and a shift in the balance of the microflora.
After formation, the species composition of plaque at a site is characterized by a degree of stability or balance among the component species, in spite of regular minor environmental stresses after periodic oral hygiene, food intake, diurnal changes in saliva flow, etc. This stability (termed microbial homeostasis) is not caused by any biologic indifference among the resident organisms, but by a balance imposed by numerous microbial interactions, including examples of both synergism and antagonism.9 These interactions include conventional biochemical ones, such as those necessary to catabolize complex host glycoproteins and to develop food chains, as well as more sophisticated cell-to-cell signaling involving intermicrobial communication via the secretion of small, diffusible molecules.10
Perturbations to Dental Plaque
In any ecosystem, the microbial homeostasis associated with normality can break down when a substantial change in a parameter that is critical to maintaining ecologic stability at a site results in the outgrowth of previously minor components of the community. A clinical consequence of this breakdown in the mouth can be disease. Significant parameters regulating homeostasis in the mouth include the integrity of the host defenses (including saliva flow) and the composition of the diet. Patients whose diet involves the regular consumption of snacks with high fermentable sugar content have greater proportions of mutans streptococci and Lactobacilli in their plaque. The biochemical properties of these bacteria mean that, in addition to being able to metabolize sugars rapidly and generate a low pH, they also are able to thrive under the acidic conditions they generate.
Dental Plaque and Caries
Numerous studies have been undertaken to determine the composition of the plaque microflora from diseased sites to try to identify those species responsible for demineralization. Interpretation of the data from such studies is difficult because plaque-mediated diseases occur at sites with a pre-existing diverse resident microflora and the traits associated with cariogenicity (acid production, acid tolerance, intracellular and extracellular polysaccharide production) are not restricted to a single species. A comparison of the properties of strains representing several streptococcal species has shown considerable overlap in the expression of these cariogenic traits.11 Similarly, the consequence of acid production by cariogenic species can be ameliorated by the development of food chains with other plaque bacteria such as Veillonellae (which convert lactate to weaker acids) or by alkali production (eg, ammonia generation from arginine metabolism) from neighboring organisms.
Despite all this interplay, clinical studies have shown caries is associated with increases in the proportions of acidogenic (acid-producing) and aciduric (acid-tolerating) bacteria, especially mutans streptococci (such as Streptococcus mutans and Streptococcus sobrinus) and Lactobacilli, which are capable of demineralizing enamel,12-14 and bifidobacteria are also being recognized as potential cariogenic bacteria in advanced lesions. These bacteria can metabolize dietary sugars to acid rapidly, creating a low pH locally. These organisms grow and metabolize optimally at low pH; under such conditions, they become more competitive, whereas most species associated with enamel health are sensitive to acidic environmental conditions. However, although mutans streptococci are implicated strongly with caries, the association is not unique; caries can occur in the apparent absence of these species, while mutans streptococci can persist without evidence of detectable demineralization. In such circumstances, some acidogenic, nonmutans streptococci are implicated with disease.12,15,16 Detailed studies of the glycolytic activity of a large number of oral streptococci have shown that some strains of nonmutans streptococci (eg, Streptococcus mitis biovar 1 and Streptococcus oralis) can still metabolize sugars to acid at a moderately low environmental pH at rates comparable with those achieved by mutans streptococci.9
These findings suggest that caries prevention strategies targeting only specific bacteria will be only partially successful, whereas approaches that reduce acid production and maintain plaque pH around neutrality will be more generally applicable.
Source of Cariogenic Pathogens
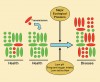
Most pathogens associated with conventional medical infections are not normally found in the host in health.4 They are acquired exogenously by ingestion, inoculation, inhalation, or direct contact. In caries, early studies using conventional bacterial culturing techniques often failed to recover putative pathogens from healthy sites or, when present, these suspect bacteria comprised only a small proportion of the microflora. However, the recent application of more sensitive molecular techniques has led to the frequent detection of low levels of mutans streptococci at a wide range of healthy sites. Bacterial typing schemes have shown that identical strains of putative cariogenic bacteria can be found in the plaque of mothers (or other close caregivers) and infants,17 implying vertical transmission of such bacteria. In either situation (ie, natural low levels of “pathogens” or low levels of exogenously acquired “pathogens”), these species would have to outcompete the already established species to achieve the levels that would enable disease to occur. As noted earlier, for dominance to happen, the normal homeostatic mechanisms that result in the maintenance of a healthy oral microflora would need to be disrupted, which is only likely to occur if there is a major ecologic disturbance to the local habitat, eg, by a substantial change in one of the key parameters (nutrition, pH) that influence or regulate the competitiveness of the oral microflora (Figure 1). This scenario suggests that plaque-mediated diseases are caused by changes in the resident microflora resulting from the selection or enrichment of the “pathogens” within the microbial community because of the imposition of strong selective pressures. Interference with these driving forces will inhibit the selection of pathogens and thereby reduce disease incidence and severity.
Factors Responsible for the Disruption of Microbial Homeostasis
Studies of a range of habitats have given clues as to the type of factors capable of disrupting the intrinsic homeostasis that exists within microbial communities. A common feature is a significant change in nutrient status, such as the introduction of a novel substrate, or in a major chemical perturbation to the site. For example, nitrogenous fertilizers washed off farmland into lakes and ponds can promote overgrowth of algae, which consume dissolved oxygen in the water leading to the loss of aerobic microbial plant and insect life (eutrophication). Similarly, atmospheric pollution with sulphur dioxide and nitrogen oxides can result in acid rain causing damage to plants, trees, and aquatic life.
Caries is associated with the frequent intake of fermentable carbohydrates in the diet, and hence plaque is exposed more often, and for longer periods, to low pH. The impact of these dramatic changes in environment on the composition of oral microbial communities has been simulated in modeling studies in the laboratory. These studies led to the formulation of an alternative hypothesis relating the role of oral bacteria to dental caries.
Impact of Environmental Change: Mixed Culture Modeling Studies
As stated earlier, individuals who frequently consume sugar in their diet generally have elevated levels of cariogenic bacteria such as mutans streptococci and Lactobacilli in their plaque and are at greater risk of dental caries. In animal studies or epidemiologic surveys of humans, it can never be determined whether the rise in cariogenic bacteria is caused by the sudden availability of sugar per se (eg, because of more efficient sugar transport systems in these bacteria) or a response to the inevitable conditions of low pH after sucrose (a molecule consisting of glucose and fructose) consumption. However, the exploitation of the unique benefits of a sophisticated system for growing bacteria under conditions in which key parameters can be controlled, but varied independently of other factors (chemostat culturing), coupled with the reproducibility of a defined mixed culture inoculum, enabled the linked effects of glucose and low pH to be separated for the first time.18 Two chemostats were inoculated with nine or ten species (representative of those found in enamel health and caries) in a growth medium in which mucin was the main source of carbohydrate and were maintained at a constant pH of 7. The cariogenic bacteria (S mutans and Lactobacillus rhamnosus) were noncompetitive under these conditions and made up < 1% of the total microflora ((Table 1). Both chemostats then were pulsed daily for 10 consecutive days with glucose. In one chemostat, the pH was maintained automatically throughout the study at neutral pH (as is found in the healthy mouth) to determine the effect of a fermentable sugar on culture stability, while in the other, the pH was allowed to fall by bacterial metabolism for 6 hours after each pulse (as occurs in vivo); the pH then was returned to neutrality for 18 hours before the next pulse.18 Daily pulses of glucose at a constant pH of 7 had little or no impact on the balance of the microbial community, and the combined proportions of S mutans and L rhamnosus stayed at approximately 1% of the total microflora ((Table 1). In contrast, when the pH was allowed to change after each pulse, there was a progressive selection of the cariogenic (and acid-tolerating) species at the expense of bacteria associated with dental health. After the final glucose pulse, the community was dominated by species implicated in caries (S mutans and L rhamnosus comprised approximately 55% of the microflora).18 Significantly, when the pH was returned to a pH of 7 after the period of uncontrolled acid production, the composition of the consortium recovered and health-associated species increased in proportion again.
When this study was repeated, with the pH fall restricted after each glucose pulse to a pH of 5.5, 5, or 4.5 in independent experiments,19 a similar enrichment of cariogenic species at the expense of healthy species was observed, but their rise was directly proportional to the terminal pH (Table 1). These studies showed unequivocally that the low pH generated from glucose metabolism rather than glucose availability led to the breakdown of microbial homeostasis in dental plaque and that a plaque pH of around neutrality favored the growth of bacteria associated with sound enamel. These laboratory findings supported earlier observations in which increases in the proportions of mutans streptococci in plaque occurred when volunteers rinsed repeatedly with low pH buffers.20 These findings have important implications for caries control and prevention; the data suggest the selection of cariogenic bacteria could be prevented if the pH changes resulting from glucose metabolism are reduced.
Current Hypotheses to Explain the Role of Plaque Bacteria in the Etiology of Dental Caries
Two main schools of thought exist on the role of plaque bacteria in the etiology of caries and periodoal diseases. The “Specific Plaque Hypothesis” proposed that out of the diverse collection of organisms comprising the resident plaque microflora, only a few species are involved in disease activity.21 This proposal focused on controlling disease by targeting preventative measures and treatment against a limited number of organisms. In contrast, the “Non-Specific Plaque Hypothesis” considered that disease is the outcome of the overall activity of the total plaque microflora.22 In this way, a heterogeneous mixture of microorganisms could play a role in disease. In some respects, the arguments about the relative merits of these hypotheses may be about semantics because plaque-mediated diseases are essentially mixed culture (polymicrobial) infections, in which only a limited (perhaps specific) number of species are able to predominate.
More recently, another hypothesis has been proposed (the “Ecological Plaque Hypothesis”) that reconciles the key elements of the earlier two hypotheses.9 The data from the studies described previously suggest dental caries is a consequence of an imbalance in the resident microflora because of the enrichment within the microbial community of these “oral pathogens” caused by frequent conditions of low pH. Potentially cariogenic bacteria are found naturally in dental plaque, but these organisms are only weakly competitive at neutral pH and are present, therefore, as a small proportion of the total plaque community. In this situation, with a conventional diet, the levels of such potentially cariogenic bacteria appear clinically insignificant and the processes of demineralization and remineralization are in equilibrium. If the frequency of fermentable carbohydrate intake increases, longer intervals of low pH persist leading to enamel demineralization (approximately a pH of 5.5).23 The effect of low pH on the microbial ecology of plaque is twofold. First, low pH most favors the proliferation of acid-tolerating (and acidogenic) bacteria (including mutans streptococci and Lactobacilli), while encouraging tooth demineralization (Figure 2). Greater numbers of bacteria such as mutans streptococci and Lactobacilli in plaque result in acid being produced at faster rates, thereby enhancing demineralization further. Other bacteria also may produce acid under similar conditions yet at a slower rate11 but may be responsible for the initial stages of demineralization, or could cause frank lesions even in the absence of more aggressive cariogenic species in a susceptible host. If aciduric species were not present initially, then the repeated conditions of low pH coupled with the inhibition of competing organisms may increase the likelihood of successful colonization by mutans streptococci or Lactobacilli. This general and variable sequence of events helps explain the microbial etiology of caries and the pattern of bacterial succession observed during lesion progression in many clinical studies.
Key features of this hypothesis are that (a) the selection of “pathogenic” bacteria is coupled with changes in the environment directly (Figure 2 ) and (b) diseases need not have a specific etiology; any species with relevant traits could contribute to the disease process. Thus, mutans streptococci are among the best adapted organisms to the cariogenic environment (high sugar/low pH), but such traits are not unique to these bacteria. Strains of other species share some of these properties and, therefore, may contribute to enamel demineralization.11,15,16 A key element of the ecologic plaque hypothesis is that disease can be prevented not only by targeting the putative pathogens directly, eg, by antimicrobial or anti adhesive strategies, but also by interfering with the selection pressures responsible for their enrichment.9
Strategies consistent with the prevention of caries via the principles of the Ecological Plaque Hypothesis, and which could augment conventional effective oral hygiene practices, include:
- Inhibition of plaque acid production, eg, by fluoride containing products or other metabolic inhibitors such as xylitol. Fluoride not only improves enamel chemistry but also inhibits several key enzymes, especially those involved in glycolysis and in maintaining intracellular pH.24 Fluoride and xylitol can reduce the pH fall following sugar metabolism, and in so doing, prevent the establishment of conditions that favor growth of acid tolerating cariogenic species25,26 (Table 1). Xylitol can interfere with sugar transport in mutans streptococci, and therefore cannot be metabolized to acid nor generate a low pH in plaque.
- Limitation of foods and drinks containing fermentable sugars and/or promotion of consuming foods/drinks with nonfermentable sugar substitutes, such as aspartame or polyols (eg, sorbitol, xylitol), thereby reducing repeated conditions of low pH in plaque.
- Stimulation of saliva flow after main meals, eg, by sugarfree gum. Saliva will introduce components of the host defenses, increase buffering capacity, remove fermentable substrates, promote remineralization, and help return the pH of plaque to resting levels more quickly.
- Other approaches designed to maintain plaque pH at natural levels around neutrality.
Clinical Implications
Understanding the role of microorganisms in dental caries requires a paradigm shift away from concepts that have evolved from studies of classical medical infections with a simple and specific (eg, single species) etiology to an appreciation of ecologic principles. The development of plaquemediated disease at a site may be a breakdown of the homeostatic mechanisms that normally maintain a beneficial relationship between the resident oral microflora and the host. When assessing treatment options, an appreciation of the ecology of the oral cavity will enable the enlightened clinician to take a more holistic approach and consider the nutrition, physiology, host defenses and general well-being of the patient, as these will affect the balance and activity of the resident oral microflora. Future episodes of disease will occur unless the cause of any breakdown in homeostasis is recognized and remedied. For example, a side effect of many medications is a reduction in saliva flow. This reduction can affect glucose clearance and buffering adversely, thereby favoring the growth of acid-tolerating and potentially cariogenic bacteria. Identification of such biochemical choke points may lead to the selection of appropriate caries preventive strategies that are tailored to the needs of individual patients. In this way, the clinician would not only treat the results of the caries process but also attempt to identify and interfere with the factors that, if left alone, will inevitably lead to additional disease.
References
1. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology 2000. 2002;28:12-55.
2. Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38(3):204-211.
3. Marsh PD. Role of the oral microflora in health. Microb Ecol Health Dis. 2000;12(3):130-137.
4. Wilson M. Microbial Inhabitants of Humans. Their Ecology and Role in Health and Disease. 1st ed. Cambridge, United Kingdom: Cambridge University Press, 2005.
5. Wilks M. Bacteria and early human development. Early Hum Dev. 2007;83(3):165-170.
6. Allison DG, Gilbert P, Lappin-Scott HM, et al, eds. Community Structure and Co-operation in Biofilms. 1st ed. Cambridge, United Kingdom: Cambridge University Press, 2000;65-85.
7. Gilbert P, Maira-Litran T, McBain AJ, et al. The physiology and collective recalcitrance of microbial biofilm communities. Adv Microb Physiol. 2002;46:202-256.
8. Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721-5732.
9. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(Pt 2):279-294.
10. Kolenbrander PE, Andersen RN, Blehert DS, et al. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66(3):486-505.
11. de Soet JJ, Nyvad B, Kilian M. Strain-related acid production by oral streptococci. Caries Res. 2000;34(6):486-490.
12. Marsh PD. Microbiologic aspects of dental plaque and dental caries. Dent Clin North Am. 1999;43(4):599-614.
13. Bowden GH. Microbiology of root surface caries in humans. J Dent Res. 1990;69(5):1205-1210.
14. Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40(3):1001-1009.
15. Sansone C, van Houte J, Joshipura K, et al. The association of mutans streptococci and non-mutans streptococci capable of acidogenesis at a low pH with dental caries on enamel and root surfaces. J Dent Res. 1993;72(2):508-516.
16. Brailsford SR, Shah B, Simons D, et al. The predominant aciduric microflora of root-caries lesions. J Dent Res. 2001; 80(9):1828-1833.
17. Tanner AC, Milgrom PM, Kent R Jr, et al. Similarity of the oral microbiota of pre-school children with that of their caregivers in a population-based study [published erratum appears in: Oral Microbiol Immunol. 2003;18(5):338]. Oral Microbiol Immunol. 2002;17(6):379-387.
18. Bradshaw DJ, McKee AS, Marsh PD. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68(9):1298-1302.
19. Bradshaw DJ, Marsh PD. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res. 1998;32(6): 456-462.
20. Svanberg M. Streptococcus mutans in plaque after mouth-rinsing with buffers of varying pH values. Scand J Dent Res. 1980;88(1):76-78.
21. Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65-107.
22. Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. 1986;13(10):905-911.
23. Zero DT, Moynihan P, Lingstöm P, et al. The role of dietary control. In: Fejerskov O, Kidd E, eds. Dental Caries. The Disease and its Clinical Management. 2nd ed. Oxford, United Kingdom: Blackwell Munksgaard; 2008:329-352.
24. Marquis RE, Clock SA, Mota-Meira M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 2003;26(5):493-510.
25. Bradshaw DJ, Marsh PD, Hodgson RJ, et al. Effects of glucose and fluoride on competition and metabolism within in vitro dental bacterial communities and biofilms. Caries Res. 2002;36(2):81-86.
26. Bradshaw DJ and Marsh PD. Effect of sugar alcohols on the composition and metabolism of a mixed culture of oral bacteria grown in a chemostat. Caries Res. 1994;28(4):251-256.