You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The association between waterborne microorganisms and human disease caused by contamination of water sources has been documented since the mid-1850s.1 Since then, a wide variety of both microscopic and macroscopic forms have been shown to survive and proliferate in both natural water and manmade environmental systems (Table 1).2 Although established public health water-treatment regulations (ie, filtration and chlorination) have been in effect for many years, waterborne infection and disease outbreaks continue to be reported from drinking water, recreational water, hospital water, and water from healthcare-related devices. Extensive documentation of these types of infections can be found in medical literature.3-8 Until recently, outbreaks and severe infections from contaminated water in healthcare settings were primarily reported in hospitals.
To put this public health issue into perspective for dentistry, it was not until 1963 that Blake first reported high concentrations of bacterial accumulation in coolant water for high-speed dental handpieces.9 Later investigations established that microorganisms in dental water-delivery systems could form biofilms leading to proliferation of very high concentrations of bacteria. Subsequent studies have reported on (a) the variety and nature of detectable microbial forms; (b) mechanisms of microbial colonization in dental-unit water lines (DUWLs) to form biofilms; (c) potential infection problems heavily colonized water can present for patients and dental healthcare providers; (d) possible approaches for reducing microbial burden and maintaining safe, potable water for patient care; and (e) developing test systems to monitor effectiveness of water-treatment procedures.10-15
Most microorganisms detected in dental water systems originate from the public water supply and do not usually present a high risk of disease for healthy dental patients. However, multiple bacteria normally isolated from colonized dental-unit water have the potential to cause infection and illness in patients with immune-compromising conditions. The infection control challenge posed by contaminated DUWLs shifted in recent years from the category of "potential" to "documented" infection. Since 2012, one death due to Legionella pneumophila16 and two outbreaks ofMycobacterium abscessus infections have been reported among pediatric dental patients after treatment with dental water that was heavily colonized with bacteria.16-18 Fortunately, continued progress to address this emerging problem has led to development of multiple approaches aimed at reducing dental-water colonization from environmental and human sources.
As more dental professionals adopt and routinely use available water-treatment technologies, many are asking questions concerning testing and looking for assurance that products and procedures are being used correctly. Among the questions:
• What water-monitoring systems are available to test microbial loads in dental water?
• Do these monitoring systems provide accurate and useful information concerning effectiveness of practice treatment procedures?
• What are appropriate aseptic procedures when collecting water samples for testing?
These timely, appropriate questions serve as the major topics for the following discussion.
The Centers for Disease Control and Prevention, American Dental Association, and other public health agencies recommend that dental-treatment water meets the US Environmental Protection Agency (EPA) drinking-water standard of less than 500 colony-forming units per milliliter (CFU/mL) of non-coliform heterotrophic bacteria for routine (nonsurgical) procedures.19-21 Dental practices have been working to meet this microbial threshold by using a combination of procedures, which involve the following:
• use of antimicrobial chemicals and technologies to control microbial colonization of DUWLs
• flushing lines in the beginning of the day and after each patient and emptying water lines overnight
• periodic application of DUWL "shock" treatments to augment routine treatment protocols
Basics of Water-Line Testing
Every dental facility is responsible for meeting the minimum DUWL standards to ensure patient and dental-worker safety. Testing water lines is the most reliable way to confirm and document that dental-water quality is acceptable. Many variables affect dental-water quality and test results, and all testing methods currently used for dental-water testing are inherently limited. All heterotrophic plate count (HPC) methods reveal only a fraction of microorganisms in any water sample-no single method will recover all microorganisms.21 Dental-water testing is expected to detect elevated numbers of a spectrum of representative heterotrophic species for the purpose of detecting failure, or confirming success, of water-line treatment and management.
Water-line testing captures and assesses waterborne species of planktonic heterotrophic bacteria at one point in time to estimate the amount of contamination within the lines. Biofilm communities change rapidly, requiring repeated testing to reliably monitor water quality over time. Many factors can affect DUWL test results, including movement of tubing, water usage and flow, cycles of bacterial growth, and water-line antimicrobial treatment.
Although scientific researchers can identify and enumerate water microorganisms with great accuracy in laboratories, such processes are costly, time-consuming, and less practical for general DUWL testing. There is a need for affordable, efficient alternatives that provide a meaningful assessment of water safety. Commercially available methods are designed to reduce cost and simplify the process by limiting parameters. Selection criteria for commercial water-testing methods should include the following considerations:
• selection for significant types of organisms (and omission of others)
• general efficacy (ability to grow and identify organisms)
• correcting for effects of residual antimi- crobial agents
• time requirements
• temperature requirements
• technique difficulty
• equipment needed
• ease of interpretation
• cost and practicality
The two basic options for water-line testing are in-office methods and mail-in water-testing services offered by commercial laboratories. In-office testing methods are designed to avoid the complexity, cost, and time required for laboratory testing methods, but they have been found to be less reliable than the standard laboratory water-testing methods used by validated laboratories.21-23 Whatever the approach used, it should be designed to detect stressed organisms typically found in water lines that are being treated with commercial antibiofilm products. These organisms are difficult to detect and grow in their starved inactive state but can proliferate in exposed susceptible hosts. Most water-line treatment products add a low-level chemical to the dental-treatment water to lower CFUs. Any residual antimicrobial chemical may affect test results by inhibiting bacterial growth during testing. In-office tests use water (with antimicrobial agents) directly from dental tubing without dilution or filtration, whereas recommended laboratory test methods filter and serially dilute samples to reduce the effects of the antimicrobial products, allowing detection of these organisms.23
In-Office Water-Line Testing
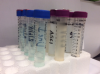
In-office water-line tests include Aquasafe® (HPTC, hptcinc.com), available from dental supply companies; an in-office test is also available as HPC Sampler from EMD Millipore (emdmillipore.com). The HPC sampler (Figure 1) is a dip paddle containing a 0.45-µm filter and an absorbent pad with dehydrated agar medium within a plastic case. Dental-unit water is poured in the case, and then the paddle is dipped into the water. The paddle absorbs 1 mL of the liquid sample; the remaining liquid is discarded. The paddle is incubated at room temperature for 7 days. The manufacturer states that accurate readings are possible up to 300 CFU/mL. Over this amount the colonies grow together (confluent growth), with the results too numerous to count (TNTC).
There is evidence that the in-office method underestimates water contamination.21 Researchers identify two key reasons for poor growth of DUWL heterotrophic bacteria on HPC samplers: variation from room temperature (22° to 28°C) and omission of neutralization of the test sample. Dental-water samples that contain residual antimicrobial agents should be neutralized to provide accurate results. However, HPC samplers may be considered a useful gross screening method in primarily low-contamination situations, such as well-maintained and treated dental units.21,23,24
In-office testing places the burden of accuracy on office personnel, requiring training and dedication of time, space, and effort. Performing testing protocol using aseptic technique and adhering to the optimal bacterial-growth schedule can pose a realistic challenge to dental teams. In addition, water-line testing records and results should be kept as part of the written safety program. With these considerations, regular in-office testing can provide a rough estimate of bacterial load and identify overgrowth events during ongoing water-line management procedures.
Mail-in Water-Line Testing Processes
Professional laboratories control many of the variables in the testing process, which improves the reliability of their results. Laboratories employ trained technicians who work under controlled conditions with regulated equipment. Standard Method 9215C R2A plating methodology is considered the gold standard for enumerating common heterotrophic bacteria in potable water. Standard Method 9215D adds filtration and dilution steps to neutralize water-line antimicrobials. Mail-in laboratory testing services that use these serial plating processes with low nutrient R2A agar, low incubation temperatures, and 5 to 7 days of incubation time yield a more accurate (higher) plate count than in-office methods.21,24,25 Sterile vials, a cold pack, and a box are provided by the laboratory, and sample collection and shipping are managed by the customer. Samples must also be shipped overnight, and results are usually ready after a week of incubation (Figure 2).
Dental facilities should attempt to avoid the common errors that undermine water-line management efforts by using commercially available water-line antimicrobial products with recommended shocking procedures, carefully following manufacturer's instructions, and testing their water lines. Different lines in dental units have varied potential for biofilm accumulation and should be tested accordingly. Mail-in laboratories provide supplies and instructions along with guidance and consulting to assist offices in reaching recommended water-line safety standards. Evidence shows that after frequent (quarterly) testing protocol is established, results improve to more than a 90% pass rate (ProEdge, unpublished data).
Why and When Should Dental Water Lines Be Tested?
The recommendation to test DUWLs is targeted towards verifying the effectiveness of existing water-line management programs and confirming continual water potability. Unfortunately, errors may occur when dental workers have undetected problems in their efforts to control water-line biofilms, thereby leading to failure of management procedures. Researchers previously reported audits of DUWLs showing that up to 50% of treated lines failed to meet potable standards. These researchers suggested monthly testing protocol.23
To provide more information about dental offices that currently test their water lines, two dental-water testing laboratories, Sterilization Assurance Service (SAS) at Loma Linda University and ProEdge Dental Water Labs, were consulted. ProEdge Dental Water Labs provided anonymous data from 22,196 consecutive test results, whereas both Loma Linda University and ProEdge Dental Water Labs provided generalized assessments based on 10 years of water testing. These water-line testing results were evaluated, comparing pass and fail rates based on the following: (1) various types of devices or lines, (2) water-line antimicrobial product used (or named by the dental office), and (3) number of tests performed (ProEdge, unpublished data).
Pass Rate by Device
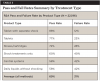
One analysis compared test results from samples taken at different devices or lines. The results showed that 76.25% of air/water syringe hoses passed and 75.84% of the handpiece hoses passed, but only 60.68% of scaler hoses passed. Average pass rate for all types of lines was 74.5% (Table 2).
Although every DUWL should be tested, testing every port may be costly and time intensive. Instead, strategies for selecting representative ports or determining sequences for rotational testing protocol have been suggested, especially after repeated testing shows acceptable water quality (ProEdge, unpublished data; M. Rust, personal communication, January 2018). If a rotational testing protocol is considered, it may be advisable to test infrequently used lines as well as frequently used lines. Pooled samples from all water-bearing lines of each unit may also be tested. If contamination is detected, lines should be re-treated and retested, possibly including individual line samples (S. Mills, personal communication, April 2018).
Pass Rate by Product Type
In addition to the testing for different types of water lines, various treatment protocols were compared. Table 3 represents field data accumulated from hundreds of dental facilities. The data reflect the potential misuse of the individual products. Failed water tests are typically corrected after identifying mistakes such as not following manufacturer instructions and not shocking on a regular basis.
The data show that periodic shocking combined with daily antimicrobial treatment yielded the highest pass rate (88%) and appears to be the most reliable long-term protocol. The average pass rate for all water that was treated with one of the above commercially available DUWL treatment products was found to be 70%. Shocking alone, without continuous water-line treatment, resulted in a 60% pass rate; using a daily treatment product without shocking yielded a 58% pass rate.
Although there are numerous options for water-line daily use cleaners, there are only two main options for shocking treatments: bleach (diluted 1:13 for a maximum 10-minute contact time) and commercially available shock products designed specifically for shocking of DUWL (left in lines overnight according to manufacturer's directions). Scientific literature supports use of both options; however, bleach is more likely to damage dental-unit materials over time (M. Rust, personal communication, January 2018; S. Mills, personal communication, April 2018). Using bleach in DUWL is off-label use of the product and is inconsistent with its EPA registration (S. Mills, personal communication, April 2018).
Pass Rate by Number of Tests
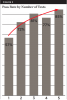
An important point made by both laboratories during interviews was that water-line samples from their customers often reveal high levels of contamination initially but that those levels improve after repeated testing. In one analysis of 3742 samples (Table 4, Figure 3), only 57% of the water samples passed (met the potable drinking water standard of 500 CFU/mL) on the first test, but results improved after repeated testing. After five repeat tests, 85% of the samples met potable standards.
Water-line testing must be continued over time because resistant biofilm species may rebound after several years of constant use of some DUWL antimicrobial products. Both laboratories offer consultation services, which they partially credit with improved water quality and test results. Improved water test results are considered confirmation that the product, equipment maintenance, protocol, technique, and compliance are successful. Keeping records of water-line test results as a record of compliance with safety standards can provide a facility with ongoing documentation of their efforts in this area (W. Zhang, personal communication, April 2018; M. Rust, personal communication, January 2018).
Understanding possible reasons for poor test results can aid in revising and improving a facility's DUWL treatment protocols. Both testing services identified key reasons for poor test results. Before beginning water-line testing, safety managers can assist in avoiding typical errors by addressing and correcting common problems. Management factors that are necessary for controlling water-line contamination and ensuring good test results include the following:
DUWL product manufacturer's directions for use must be followed. Examples are regular shock treatments, correct dilution of product, correct sequencing or frequency of water treatment, and emptying water bottles or drying lines as recommended. All DUWLs should be shocked periodically with a strong chemical to remove biofilm. Water-line products retard (but do not prevent) biofilm growth. Biofilm builds up slowly and must be removed. Shock treatments are recom- mended weekly if a continuous antimi- crobial product is not used; however, less- frequent shock procedures may be neces- sary when continuous antimicrobials are in place. Water-line testing can determine the optimal time interval for shocking as well as the need for shocking, even when manufacturers' directions exclude shock- ing instructions (W. Zhang, personal communication, April 2018; M. Rust, personal communication, January 2018; S. Mills, personal communication, April 2018).
Source water should be clean. Distilled water, in-office distillers, and reverse-osmosis units may become contaminated over time. Filtered or processed water should be tested because filters may become contaminated. Municipal tap water may contain organisms that prolif- erate in DUWLs, and "hard" water with high total dissolved solids (TDS) can neutral- ize water-line treatment products. These contaminants in source water can exhaust active ingredients of antimicrobial products before water-line microbes are neutralized. It should be noted that TDS filters do not filter out microorganisms.
DUWLs should be flushed at the beginning and end of the day and between patients. Flushing is recommended to remove contaminated fluids after periods of stagna- tion and after possible retraction of patient- derived contaminants.
Shocking should not be confused with flushing processes. Shocking is the process of treating DUWLs with strong chemicals that detach biofilm from the internal surfaces of water lines. Flushing is running treatment water through open ports of DUWLs, which removes fluids but will not reliably remove attached biofilms.
DUWLs should be tested consistently to confirm maintenance-protocol effectiveness and determine proper shock frequency.
Summary
Multiple approaches are available for treating dental water. When used appropriately, these treatments can aid facilities in meeting regulated water standards. As dental professionals continue to become better acquainted and more comfortable with the different options, an important quality control measure is the periodic testing of DUWLs. Testing is the most reliable way to discover problems with compliance and also provides documentation of dental-water quality. Test kits for in-office use and mail-in testing services already provide means for dental facilities to assess the effectiveness of their treatment protocols. These products and services may become more user-friendly as new equipment management options and test methods emerge. Nevertheless, dental professionals should understand how to choose and correctly perform DUWL testing.
About the Authors
Nancy Dewhirst, RDH, BS
DentalEducator/Consultant/Writer, Professor, West Coast University, Anaheim, California
John A. Molinari, PhD
Professor Emeritus, University of Detroit Mercy, School of Dentistry, Detroit, Michigan; Director of Infection Control, Dental Advisor, Ann Arbor, Michigan
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Snow J. On the mode of communication of cholera. In: Clendening L, ed. The Sourcebook of Medical History. New York, NY: Paul B. Hoeber, Inc.; 1942:468-472.
2. Molinari J. The ongoing challenge of waterborne infections. Inside Dent. 2017;13(8):52-58.
3. Craun GF, Brunkard JM, Yoder JS, et al. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin Microbiol Rev. 2010;23(3):507-528.
4. Hlavsa MC, Roberts VA, Kahler AM, et al. Outbreaks of illness associated with recreational water-United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):668-672.
5. Rutala WA, Weber DJ. Water as a reservoir of nosocomial pathogens. Infect Control Hosp Epidemiol. 1997;18(9):609-616.
6. Decker BK, Palmore TN. The role of water in healthcare-associated infections. Curr Opin Infect Dis. 2013;26(4):345-351.
7. Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62(11):1423-1435.
8. McClung RP, Roth DM, Vigar M, et al. Waterborne disease outbreaks associated with environmental and undetermined exposures to water-United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66(44):1222-1225.
9. Blake GC. The incidence and control of bacterial infection of dental units and ultrasonic scalers. Brit Med J.1963;115:413-416.
10. Martin MV. The significance of the bacterial contamination of dental unit water systems. Brit Dent J. 1987;163(5):152-154.
11. Williams JF, Johnston AM, Johnson B, et al. Microbial contamination of dental unit water lines: prevalence, intensity, and microbial characteristics. J Am Dent Assoc. 1993;124(10):59-65.
12. Karpay RI, Plamondon TJ, Mills SE, et al. Comparison of methods to enumerate bacteria in dental unit water lines. Curr Microbiol. 1999;38(2):132-134.
13. Mills SE. The dental unit water line controversy: defusing the myths, defining the solutions. J Am Dent Assoc. 2000;131(10):1427-1441.
14. Mills SE. Dental unit water and air quality challenges. In: Molinari JA, Harte JA, eds. Cottone's Practical Infection Control in Dentistry. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2010:63-75.
15. Gruninger SE. Disease transmission through dental unit water: an update. ADA Professional Product Rev. 2014;9(2):8.
16. Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit water line. Lancet.2012;379(9816):684.
17. Peralta G, Tobin-D'Angelo M, Parham A, et al. Notes from the field: mycobacterium abscessus infections among patients of a pediatric dentistry practice - Georgia, 2015. MMWR Morb Mortal Wkly Rep.2016;65(13):355-356.
18. American Dental Association. Nontuberculous mycobacterial infection linked to pulpotomy procedures and possible dental water line contamination reported in California and Georgia. https://www.ada.org/en/science-research/science-in-the-news/nontuberculosis-mycobacterial-infection-linked-to-pulpotomy-procedures. Published September 21, 2016. Accessed May 15, 2018.
19. Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep.2003;52(RR-17):1-61.
20. Kohn WG, Harte JA, Malvitz DM, et al. Guidelines for infection control in dental health care settings-2003. J Am Dental Assoc. 2004;135(1):33-47.
21. Porteous N, Sun Y, Schoolfield J. Evaluation of 3 dental unit water line contamination testing methods. Gen Dent. 2015;63(1):41-47.
22. Cohen ME, Harte JA, Stone ME, et al. Statistical modeling of dental unit water bacterial test kit performance. J Clin Dent. 2007;18(2):39-44.
23. Lal S, Singhrao SK, Brickness M, et al. Monitoring dental-unit-water-line output water by current in-office test kits. Curr Microbiol.2014;69(2):135-142.
24. Momeni SS, Tomline N, Ruby JD, Dasanayake AP. Evaluation of in-office dental unit water line testing. Gen Dent. 2012;60(3):e142-e147.
25. Reasoner DJ. Heterotrophic plate count methodology in the United States. Int J Food Microbiol.2004;92(3):307-315.