You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Unquestionably, the aging dental patient population is consuming more and more drugs, including a variety of psychotropic medications and cardiovascular drugs.1 The most common drugs that dentists prescribe or administer include nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen (Table 1), antibiotics and antifungals such as metronidazole (eg, Flagyl®) and fluconazole (eg, Diflucan®), and local anesthetics containing the vasoconstrictor epinephrine (Table 2). What many clinicians do not realize is that these commonly employed drugs in practice can be involved in serious adverse drug interactions with medications patients are taking for a variety of medical conditions. This article will review three of the serious interactions that can potentially occur within the practice of dentistry.
NSAIDs And Lithium
As illustrated in Table 1, there are a variety of NSAIDs from which dentists can choose to manage odontogenic and postoperative pain. These analgesics represent the first line drugs that should be employed in this situation because of their unique mechanism of action, an inhibition of prostaglandin synthesis at the site of surgical trauma, which renders these drugs highly effective in the treatment of postoperative dental pain.2,3 There are numerous evidence-based, double-blind, placebo-controlled published studies that demonstrate the overall effectiveness of these drugs after the surgical removal of impacted third molars.4-11 However, in certain patients, NSAIDs should be avoided or used cautiously because of the possibility of precipitating a serious adverse drug interaction. A comprehensive review of this subject can be found in previous publications.12,13 One such drug is lithium.14
Lithium is a major remedy in the treatment of bipolar depressive disorder.15 It has a low therapeutic index, which means the difference between effective doses and toxic doses is relatively small. Therefore, plasma levels of lithium must be carefully monitored to ensure therapeutic effectiveness while avoiding toxicity.15 The NSAIDs inhibit the renal excretion of lithium and can cause plasma lithium to accumulate to toxic levels, potentially leading to renal, gastrointestinal, and central nervous toxicity.14-18 Both ibuprofen 1800 mg/day and naproxen 750 mg/day for 6 days have been demonstrated to increase previously stable lithium plasma levels, and the magnitude of this effect varied widely among individuals.16,17 Ibuprofen produced a mean increase of 34% (range 12% to 66%), while naproxen produced a mean increase of 16% (range 0% to 42%). Individual cases of three- to four-fold increases in lithium blood levels accompanied by stupor, ataxia, confusion, and renal failure have been reported after the use of ibuprofen 1600 mg/day for 1 week to treat shoulder pain.18 A more recent report describes a 51-year-old patient with a history of bipolar disorder on lithium therapy presenting to an emergency department with confusion, dysarthria, abnormal gait, and diarrhea.19 He subsequently needed to be intubated before being discharged from the hospital. His symptoms started 2 days after his dentist prescribed ibuprofen 800 mg 3 times daily after extracting an infected molar. His laboratory values were significant for elevated lithium levels of 3 mmol/liter (therapeutic range 0.6 mmol/liter to 1 mmol/liter) and mild renal failure. Another recent report describes a 49-year-old woman with stable lithium concentrations experiencing lethargy, diarrhea, nausea, vomiting, hypersalivation, tremors, muscle weakness, and confusion 3 days after being started on the NSAID meloxicam. Her serum lithium levels were greater than 5 mmol/liter.20
It is recommended that before prescribing NSAID analgesics to a patient on lithium therapy, dentists consult with the patient’s psychiatrist. More frequent lithium blood level monitoring (every 4 to 5 days) should be initiated, and reductions in the lithium dose may be required.14 An alternative is to avoid NSAID analgesics altogether in lithium-treated patients and prescribe acetaminophen, or if necessary, an acetaminophen/opioid combination drug such as acetaminophen plus hydrocodone (eg, Vicodin®).
Metronidazole or Fluconazole in Combination with Warfarin
Metronidazole is highly effective against obligate anaerobic bacteria associated with periodontal disease, periapical abscesses, and peri-implantitis.21-24 Because it has no activity against facultative anaerobic bacteria, which may be part of a flora mixture inhabiting these infected sites, metronidazole frequently is combined with a penicillin or with ciprofloxacin.21-23 Fluconazole is an antifungal agent that is effective in the treatment of mucosal candidiasis and other candidial infections in the oral cavity.25
Warfarin (eg, Coumadin®) is the most frequently prescribed anticoagulant in the world and is employed in preventing myocardial infarctions, pulmonary embolisms, and occlusive strokes in high-risk patients, such as those with atrial fibrillation, heart valve replacement, and deep venous thrombosis.26,27 Similar to lithium, warfarin has a low therapeutic index, and monthly monitoring of patients’ coagulation status is advised to ensure that plasma levels are in the therapeutic range.12,27 Excessive blood levels of warfarin can lead to internal bleeding, including intracranial bleeding.28
Warfarin is mainly metabolized through the intestinal and hepatic cytochrome P-450 system, whose predominant metabolizing isoform is cytochrome P-450 2C9 (CYP 2C9).29 Metronidazole and fluconazole are potent inhibitors of CYP 2C9; thus, they can block warfarin metabolism and subsequently increase blood levels of warfarin to toxic levels, especially its more potent S(-) isomer.29-31
In a study of eight normal volunteers, pretreatment with metronidazole 750 mg/day for 1 week significantly increased plasma levels and half-lives of even a single dose of warfarin compared to taking warfarin alone.32 This was accompanied by a significant increase in mean prothrombin times.32 In one case report, a 31-year-old woman who had received 6 years of warfarin therapy without a previous bleeding episode was admitted to a hospital with several ecchymoses of both legs and obvious swelling and hemorrhage into the subcutaneous tissue behind her left knee after completing a 10-day course of metronidazole 750 mg/day for a trichomoniasis infection.30 Her prothrombin time was 147 seconds; the normal prothrombin time is 17 to 19 seconds. Vitamin K, the antidote for a warfarin overdose, was given and her condition resolved over 1 week.30 More recently, a 78-year-old woman was started on metronidazole 250 mg every 8 hours for 5 days, and levofloxacin (Levoquin®) 500 mg once a day for 6 days for an upper respiratory tract infection.33 The patient did not notify any of the healthcare professionals that she was on concomitant warfarin therapy. Her most recent international normalized ratio (INR) reading had been 2.5. Six days after her clinic visit, the patient was admitted to the hospital for a profuse nosebleed and “an unusual headache,” and a CAT scan revealed she had a minor hemorrhagic stroke. Her INR had risen to 8.0. After a 1-week hospital stay, which included the administration of vitamin K and a blood transfusion, she was discharged.33
Cases of cerebral hemorrhage,34 gastrointestinal bleeds,35,36 intraocular hemorrhage,37,38 and significantly elevated INRs36,39 due to a warfarin–fluconazole interaction have appeared in the literature. In a retrospective cohort study, 22,272 veterans who had been on warfarin therapy for at least 1 month were administered an antimicrobial agent. Among them, 9.7% of those who received fluconazole and 4.9% of those who received metronidazole had INRs that were greater than 6.0 (normal INRs in anticoagulated patients should be between 2.0 and 3.0).40 Employing US Medicaid data, a case-control study of 308,100 warfarin users demonstrated an elevated risk (odds ratio = 2.09) of gastrointestinal bleeding in warfarin recipients receiving fluconazole compared to those receiving the non-interacting antibiotic cephalexin (Keflex®).41
Based on these case reports and clinical studies, the authors recommend that dentists avoid prescribing metronidazole or fluconazole in patients on concomitant warfarin therapy.
Epinephrine with Propranolol
There is probably no area in dental pharmacology that is more highly debated than the use or avoidance of epinephrine-containing local anesthetics in certain medically complex patient populations, including those taking potentially interacting drugs.12,42-47 In reality, case reports describing adverse drug interactions between vasoconstrictors in dental local anesthetic solutions and potential interacting drugs are extremely rare, partly because epinephrine is currently by far the vasoconstrictor agent most widely used with local anesthetics in dentistry. While epinephrine has alpha-1 adrenergic vasoconstrictive effects on some vascular beds—most notably under the skin and mucous membranes—it also has vasodilatory effects on other vascular beds that contain predominantly beta-2 adrenergic receptors, such as those in skeletal muscle, resulting in vasodilation48 (Table 3). This opposing vasodilatory property of epinephrine limits the potential pressor effects of the drug compared to other agents like levonordefrin and norepinephrine, which have less, and in the case of norepinephrine, almost no beta-2 adrenergic activity.44,47,49
Beta adrenergic–blocking drugs, also known as beta-blockers, are widely used in the treatment of hypertension, angina, cardiac arrhythmias, and migraine headaches.50 They are classified into two groups: nonselective beta-blockers that block both beta-1 and beta-2 receptors; and cardioselective beta-blockers, which only block beta-1 receptors (Table 4). The cardioselective beta-blockers are more widely prescribed today because their lack of beta-2 adrenergic–blocking activity limits the bronchoconstrictive effects occasionally seen with nonselective beta-blockers.50 However, the nonselective beta-blocker propranolol is still widely prescribed.51
A case series describing severe hypertensive reactions in six patients on chronic propranolol therapy receiving lidocaine with epinephrine for facial plastic surgery procedures has appeared in the literature.52 In two of the cases, the patients had been administered 10 ml and 12 ml of a 1% lidocaine plus 1:100,000 epinephrine solution, respectively. This translates into the amount of epinephrine in approximately six and seven 1.7-ml dental local anesthetic cartridges. Their blood pressures rose from normal levels (120/80 and 110/70 mm Hg) to acutely hypertensive levels (200/100 and 190/110 mm Hg), with a concomitant reflex bradycardia. In a third case, a patient receiving 13 ml of a 1:200,000 epinephrine solution went into cardiac arrest and had to be resuscitated with emergency treatment, including defibrillation.52
There is only a single case report in the dental literature in which a patient, a 32-year-old woman, taking daily propranolol for hypertension and dysrhythmias received 1.5 cartridges of 2% mepivacaine plus 1:20,000 levonordefrin (a vasoconstrictor chemically related to epinephrine).53 Her systolic and diastolic blood pressures rose by 40 mm Hg and 15 mm Hg, respectively. When two cartridges of 3% mepivacaine plain were used on a subsequent visit, her blood pressure remained stable.
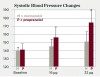
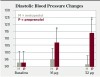
The theoretical basis of this serious adverse drug reaction between propranolol and epinephrine is that the former blocks the beta-2 vasodilatory effects of epinephrine, leaving the alpha-1 vasoconstrictive effects functioning unopposed, leading to hypertension with a concomitant reflex bradycardia.42,43,47 One of the most compelling studies supporting the adverse interaction between propranolol and epinephrine is illustrated in Figure 1 and Figure 2.54 Five patients being treated for long-standing severe hypertension with either the nonselective beta-adrenergic blocking agent propranolol or the cardioselective beta-adrenergic blocking agent metoprolol took their usual morning dose of their respective beta-adrenergic blocking agent 2 hours prior to undergoing a vasopressor challenge using slow epinephrine infusions of various doses over 8 minutes. After the completion of the first session, these patients were crossed over to the alternative treatment for at least 4 weeks, and the epinephrine challenge was administered again. As shown in Figure 1, following the slow infusion of 16 μg (2 μg/min) of epinephrine, which is slightly less than that found in a single 1.7-mL 1:100,000 epinephrine dental cartridge (17 μg or 0.017 mg),47 the mean increase in systolic blood pressure was about 15 mm Hg in the propranolol group and only 5 mm Hg in the metoprolol group. As shown in Figure 2, the differences in diastolic blood pressure following the 16-μg epinephrine infusion was even more pronounced, increasing only 2 mm Hg in the metoprolol group but 14 mm Hg in the propranolol group. This difference reached the level of statistical significance (P < 0.05). When 32 μg of epinephrine was slowly infused, an amount slightly less than two 1.7-mL cartridges of a 1:100,000 solution (34 μg or 0.034 mg),47 the metoprolol group exhibited a 10-mm Hg increase in mean systolic blood pressure, whereas the propranolol group exhibited a mean systolic blood pressure increase of 33 mm Hg (P < 0.05). Diastolic blood pressure remained unchanged in the metoprolol group but increased 21 mm Hg in the propranolol group (P < 0.05).54 Other intravenous infusion studies have reported similar pressor responses when epinephrine was administered to patients on propranolol and other nonselective beta-blockers.55-57 Although one can argue that intravenous infusions do not resemble typical submucosal dental injections, inadvertent intravascular injections do occur in dental practice, with injection speeds at least eight times more rapid (one cartridge per minute) than the infusion rates in the studies discussed here.47
There are two studies in the literature where individuals on nonselective beta-adrenergic blocking agents received dental injections of lidocaine with epinephrine.58,59 In one study, when normal volunteers were pretreated with a single oral dose of the nonselective beta-adrenergic blocking agent pindolol, small (8 mm Hg to 9 mm Hg) but significant (P < 0.05) increases in systolic and diastolic blood pressure and peripheral vascular resistance, with corresponding decreases in heart rate, were observed after the administration of two intraoral injections of 2% lidocaine plus 1:80,000 epinephrine (45 μg or 0.045 mg epinephrine total). When these same individuals were not pretreated with pindolol, the administration of the same dose of local anesthetic solution induced small decreases in systolic and diastolic blood pressure and peripheral vascular resistance.58 Similar results were reported in dental patients with cardiovascular disease on nonselective beta-blocker therapy who received a single cartridge of 2% lidocaine with 1:80,000 epinephrine (22.5 μg or 0.0225 mg epinephrine).59
Based on the case reports in the plastic surgery literature and the results of the clinical studies presented above, the following recommendations are made. In patients requiring simple restorative dentistry procedures who are on propranolol or other nonselective beta-adrenergic blocking agents, complete avoidance of local anesthetic solutions containing epinephrine, such as employing 3% mepivacaine or 4% prilocaine plain, appears prudent. In patients requiring hemostasis for dental surgical procedures or a longer duration of action, an absolute maximum of 0.034 mg of epinephrine (two cartridges of a 1:100,000 solution or four cartridges of a 1:200,000) solution is advised. Proper aspirating technique is mandatory to avoid inadvertent intravascular injections, and very slow injections rates are recommended. Before administering additional cartridges of local anesthetic solution, blood pressure and heart rate should be taken to ensure that these vital signs remain stable. The use of 1:50,000 epinephrine and the use of epinephrine-impregnated gingival retraction cord that contains 0.5 mg to 1 mg of racemic epinephrine per 2.5 cm,60 should be absolutely avoided.47,61
Conclusion
With an aging dental patient population using an increasing amount of drugs, practitioners must be cognizant of adverse drug interactions that can potentially endanger their patients. Three such serious interactions have been reviewed. NSAIDS, which are highly effective in the treatment of postoperative pain, should be avoided or used cautiously in lithium-treated patients. Prescribing either metronidazole, an effective drug against anaerobic bacteria, or the antifungal agent fluconazole should be avoided in patients who are on concomitant warfarin therapy. Finally, for patients on propranolol, epinephrine-containing local anesthetics should be avoided in patients undergoing restorative procedures of short duration and used cautiously (no more than 0.034 mg) in patients requiring hemostasis or longer duration dental procedures.
Disclosure
Within the past five years, Dr. Moore has served as medical director and/or a research consultant to several pharmaceutical companies marketing local anesthetic products, including DENTSPLY Pharmaceutical Division, Kodak Dental Systems, Septodont USA, St. Renatus, and Novocol of Canada Inc. Dr. Hersh had no disclosures to report.
About the Authors
Elliot V. Hersh, DMD, MS, PhD
Professor, Pharmacology, Department of Oral Surgery and Pharmacology, School of Dental Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
Paul A. Moore, DMD, PhD, MPH
Professor, Pharmacology and Dental Public Health, Departments of Dental Anesthesiology and Dental Public Health, School of Dental Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Heft MW, Mariotti AJ. Geriatric pharmacology. In: Yagiela JA, Dowd FJ, Johnson BS, et al, eds. Pharmacology and Therapeutics for Dentistry. 6th ed. St. Louis, MO: Mosby Elsevier; 2011:834-841.
2. Hersh EV, Kane WT, O’Neil MG, et al. Prescribing recommendations for the treatment of acute pain in dentistry. Compend Contin Educ Dent. 2011;32(3):22-30.
3. Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 2013;144(8):898-908.
4. Hersh EV, Cooper S, Betts N, et al. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76(6):680-687.
5. Fricke JR, Halladay SC, Francisco CA. Efficacy and safety of naproxen sodium and ibuprofen for pain relief after oral surgery. Curr Ther Res. 1993;54(6):619-627.
6. Kiersch TA, Halladay SC, Hormel PC. A single-dose, double-blind comparison of naproxen sodium, acetaminophen, and placebo in postoperative dental pain. Clin Ther. 1994;16(3):394-404.
7. Levin LM, Cooper SA, Betts NJ, et al. Ketoprofen dental pain study. J Clin Dent. 1997;8(4):103-106.
8. Hersh EV, Levin LM, Cooper SA, et al. Conventional and extended-release etodolac in postsurgical dental pain. Clin Ther. 1999;21(8):1333-1342.
9. Hersh EV, Levin LM, Cooper SA, et al. Ibuprofen liquigel for oral surgery pain. Clin Ther. 2000;22(11):1306-1318.
10. Zuniga JR, Noveck RJ, Schmidt WK, et al. Onset of action of diclofenac potassium liquid-filled capsules in dental surgery patients. Curr Med Res Opin. 2011;27(9):1733-1739.
11. He A, Hersh EV. A review of intranasal ketorolac tromethamine for the short-term management of moderate to moderately severe pain that requires analgesia at the opioid level. Curr Med Res Opin. 2012;28(12):1873-1880.
12. Hersh EV, Moore PA. Adverse drug interactions in dentistry. Periodontol 2000. 2008;46:109-142.
13. Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesics. Clin Ther. 2007;29(suppl):2477-2497.
14. Ragheb M. The clinical significance of lithium-nonsteroidal anti-inflammatory drug interactions. J Clin Psychopharmacol. 1990;10(5):350-354.
15. Lewis VA. Psychopharmacology: antipsychotic and antidepressant drugs. In: Yagiela JA, Dowd FJ, Johnson BS, et al, eds. Pharmacology and Therapeutics for Dentistry. 6th ed. St. Louis, MO: Mosby Elsevier; 2011:162-187.
16. Ragheb M. Ibuprofen can increase serum lithium level in lithium-treated patients. J Clin Psychiatry. 1987;48(4):161-163.
17. Ragheb M, Powell AL. Lithium interaction with sulindac and naproxen. J Clin Psychopharmacol. 1986;6(3):150-154.
18. Khan IH. Lithium and non-steroidal anti-inflammatory drugs. BMJ. 1991;302(6791):1537-1538.
19. Hassan S, Khalid F, Alirhayim Z, Amer S. Lithium toxicity in the setting of nonsteroidal anti-inflammatory medications. Case Rep Nephrol. 2013;2013:839796.
20. Chen L, Pym H. Rapid onset of neurological symptoms and lithium toxicity on starting meloxicam. Aust N Z J Psychiatry. 2010;44(1):95.
21. Ciancio SG, van Winkelhoff AJ. Antibiotics in periodontal therapy. In: Newman MG, van Winkelhoff AJ, eds. Antibiotic and Antimicrobial Use in Dental Practice. 2nd ed. Chicago, IL: Quintessence Publishing; 2001:113-126.
22. Baumgartner JC. Antibiotics in endodontic therapy. In: Newman MG, van Winkelhoff AJ, eds. Antibiotic and Antimicrobial Use in Dental Practice. 2nd ed. Chicago, IL: Quintessence Publishing; 2001:143-155.
23. Peterson LJ. Antibiotics for oral and maxillofacial infections. In: Newman MG, van Winkelhoff AJ, eds. Antibiotic and Antimicrobial Use in Dental Practice. 2nd ed. Chicago, IL: Quintessence Publishing; 2001:157-173.
24. Beikler T, Flemmig TF. Antimicrobials in implant dentistry. In: Newman MG, van Winkelhoff AJ, eds. Antibiotic and Antimicrobial Use in Dental Practice. 2nd ed. Chicago, IL: Quintessence Publishing; 2001:195-211.
25. Barchiesi F, Giacometti A, Arzeni D, et al. Fluconazole and ketoconazole in the treatment of oral and esophageal candidiasis in AIDS patients. J Chemother. 1992;4(6):381-386.
26. Nutescu EA, Shapiro NL, Ibrahim S, West P. Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin Drug Saf. 2006;5(3):433-451.
27. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
28. Tilton R, Michalets EL, Delk B, et al. Outcomes associated with prothrombin complex concentrate for international normalized ratio reversal in patients on oral anticoagulants with acute bleeding. Ann Pharmacother. 2014;48(9):1106-1119.
29. Hersh EV, Moore PA. Drug interactions in dentistry: the importance of knowing your CYPs. J Am Dent Assoc. 2004;135(3):298-311.
30. Kazmier FJ. A significant interaction between metronidazole and warfarin. Mayo Clin Proc. 1976;51(12):782-784.
31. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18(1):84-112.
32. O’Reilly RA. The stereoselective interaction of warfarin and metronidazole in man. N Engl J Med. 1976;295(7):354-357.
33. Howard-Thompson A, Hurdle AC, Arnold LB, et al. Intracerebral hemorrhage secondary to a warfarin-metronidazole interaction. Amer J Geriatr Pharmacother. 2008;6(1):33-36.
34. Isalska BJ, Stanbridge TN. Fluconazole in the treatment of candidal prosthetic valve endocarditis. BMJ. 1988;297(6642):178-179.
35. Seaton TL, Celum CL, Black DJ. Possible potentiation of warfarin by fluconazole. DICP. 1990;24(12):1177-1178.
36. Kerr HD. Case report: potentiation of warfarin by fluconazole. Am J Med Sci. 1993;305(3):164-165.
37. Mootha VV, Schluter ML, Das A. Intraocular hemorrhages due to warfarin fluconazole drug interaction in a patient with presumed Candida endophthalmitis. Arch Ophthalmol. 2002;120(1):94-95.
38. Turrentine MA. Single-dose fluconazole for vulvovaginal candidiasis: impact on prothrombin time in women taking warfarin. Obstet Gynecol. 2006;107(2 Pt 1):310-313.
39. Gericke KR. Possible interaction between warfarin and fluconazole. Pharmacotherapy. 1993;13(5):508-509.
40. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657-663.
41. Schelleman H, Bilker WB, Brensinger CM, et al. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84(5):581-588.
42. Jastak JT, Yagiela JA. Vasoconstrictors and local anesthesia: a review and rationale for use. J Am Dent Assoc. 1983;107(4):623-630.
43. Goulet JP, Pérusse R, Turcotte JY. Contraindications to vasoconstrictors in dentistry: Part III. Pharmacologic interactions. Oral Surg Oral Med Oral Pathol. 1992;74(5):692-697.
44. Yagiela JA. Adverse drug interactions in dental practice: interactions associated with vasoconstrictors. Part V of a series. J Am Dent Assoc. 1999;130(5):701-709.
45. Brown RS, Rhodus NL. Epinephrine and local anesthesia revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(4):401-408.
46. Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54(4):587-599.
47. Hersh EV, Giannakopoulos H. Beta-adrenergic blocking agents and dental vasoconstrictors. Dent Clin North Am. 2010;54(4):687-696.
48. Yagiela JA. Vasoconstrictors agents for local anesthesia. Anesth Prog. 1995;42(3-4):116-120.
49. Boakes AJ, Laurence DR, Lovel KW, et al. Adverse reactions to local anesthetic-vasoconstrictor preparations. A study of the cardiovascular responses to Xylestesin and Hostacain-with-Noradrenaline. Br Dent J. 1972;133(4):137-140.
50. Yaping T, Piacick MT, Abel PW. Adrenergic antagonists. In: Yagiela JA, Dowd FJ, Johnson BS, et al, eds. Pharmacology and Therapeutics for Dentistry. 6th ed. St. Louis, MO: Mosby Elsevier; 2011:106-116.
51. Most commonly prescribed drugs. http://www.emcp.com/college_resource_centers/listonline.php?GroupID=7240. Accessed July 18, 2014.
52. Foster CA, Aston SJ. Propranolol-epinephrine interaction: a potential disaster. Plast Reconstr Surg. 1983;72(1):74-78.
53. Mito RS, Yagiela JA. Hypertensive response to levonordefrin in a patient receiving propranolol: report of case. J Am Dent Assoc. 1988;116(1):55-57.
54. Houben H, Thien T, van’t Laar A. Effect of low-dose epinephrine infusion on hemodynamics after selective and nonselective beta-blockade in hypertension. Clin Pharmacol Ther. 1982;31(6):685-690.
55. Mackie K, Lam A. Epinephrine-containing test dose during beta-blockade. J Clin Monit. 1991;7(3):213-216.
56. Hjemdahl P, Akerstedt T, Pollare T, Gillberg M. Influence of beta-adrenoceptor blockade by metoprolol and propranolol on plasma concentrations and effects of noradrenaline and adrenaline during i.v. infusion. Acta Physiol Scand Suppl. 1983;515:45-53.
57. Rehling M, Svendsen TL, Maltbaek N, et al. Haemodynamic effects of atenolol, pindolol and propranolol during adrenaline infusion in man. Eur J Clin Pharmacol. 1986;30(6):659-663.
58. Sugimura M, Hirota Y, Shibutani T, et al. An echocardiographic study of interactions between pindolol and epinephrine contained in a local anesthetic solution. Anesth Prog. 1995;42(2):29-35.
59. Niwa H, Sugimura M, Satoh Y, Tanimoto A. Cardiovascular response to epinephrine-containing local anesthesia in patients with cardiovascular disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(6):610-616.
60. Kellam SA, Smith JR, Scheffel SJ. Epinephrine absorption from commercial gingival retraction cords in clinical patients. J Prosthet Dent. 1992;68(5):761-765.
61. Naftalin LW, Yagiela JA. Vasoconstrictors: indications and precautions. Dent Clin North Am. 2002;46(4):733-746.