You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Disclaimer: Participants must always be aware of the hazards of using limited knowledge in integrating new techniques or procedures into their practice. Only sound evidence-based dentistry should be used in patient therapy.
Introduction
This course discusses common immune-mediated and idiosyncratic adverse drug reactions (ADRs) related to the top 200 drugs dispensed by U.S. community pharmacies and less commonly noted ADRs affecting oral tissues. In addition, uncommon, time-related (delayed), usually dose-related ADRs, i.e., carcinogenesis and teratogenesis, and some secondary ADRs are presented.
Please note this is Part II of a two-part series. Adverse Drug Reactions – Part I discusses common “on-target,” “off-target,” and (3) cytotoxic reactions related to the top 200 drugs dispensed by U.S. community pharmacies, and less common ADRs that relate to dental therapeutics and/or manifest in the head and neck area.
Drugs seldom exert their beneficial effects without also causing adverse drug reactions (ADRs). Major mechanisms of ADRs include (1) “on-target” adverse reactions, (2) “off-target” adverse reactions, (3) cytotoxic reactions, (4) immune-mediated reactions, and (5) idiosyncratic reactions.1 In Adverse Drug Reactions – Part I, the discussion focused on mechanisms (1), (2), and (3) and related common ADRs that can occur with therapeutic doses of drugs in the top 200 dispensed by U.S. community pharmacies.2
In Adverse Drug Reactions – Part II, the discussion is extended to mechanisms (4) immune-mediated reactions and (5) idiosyncratic reactions, and related common ADRs that occur with drugs in the top 200 dispensed by U.S. community pharmacies and to less commonly noted ADRs affecting oral tissues. In addition, a brief discussion of the mechanisms of uncommon, time-related (delayed), and usually dose-related ADRs, i.e., carcinogenesis and teratogenesis, and some secondary ADRs are presented.
Immune-mediated Mechanisms of ADRs
There are three major categories of ADRs related to the immune system: immunotoxicity, autoimmune reactions, and hypersensitivity or allergic reactions.1 Immunotoxicity may be the specific intent of therapy, e.g., when monoclonal antibodies target specific B cells. Alternatively, it may occur as an ADR to therapy when cytotoxic agents designed to kill malignant cells also damage normal cells in the bone marrow and lymphoid tissues. Immunotoxicity may also result in secondary ADRs, e.g., infections and oncogenesis.
Drugs can also initiate autoimmune reactions resulting in a person's immune system attacking his/her own cells. For example, a drug may elicit an antibody response to Rh factors on red blood cells (RBCs) causing hemolytic anemia; or induce an antibody response to myeloperoxidase or DNA causing a lupus-like syndrome; or directly cause mast cell degranulation resulting in urticarial lesions; or cause blistering lesions of the skin and mucous membranes, e.g., erythema multiforme or Stevens-Johnson syndrome.
Hypersensitivity or allergic reactions reflect drug-related immunogenicity. Therapeutic peptides or proteins with molecular weights >600 daltons are recognized by the immune system as foreign substances and can directly trigger allergic reactions. Drugs with molecular weights <600 daltons are too small to act as direct immunogens; however, these drugs may act as haptens. Haptens bind covalently to large endogenous protein molecules and the hapten-protein complex triggers the allergic response.
The Gell-Coombs classification system proposes four mechanisms of hypersensitivity or allergic reactions: type I or immediate hypersensitivity reactions (anaphylaxis), type II or antibody-dependent cytotoxic reactions, type III or immune complex-mediated reactions (serum sickness), and type IV or delayed T cell-mediated reactions.1,3 An allergic response is predicated on sensitization, i.e., prior exposure to a drug. At highest risk are adults, women, patients with HIV infection, and those with history of allergy to related drugs.
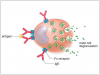
Type I or immediate hypersensitivity reactions (anaphylaxis) are predicated on exposure to an allergen and antigen-specific antibody production dominated by immunoglobulin E (IgE) isotype.1,3 Upon re-exposure IgE antibodies bind to mast cells in mucosal and epithelial tissues (Figure 1). The simultaneous binding of an antigen to adjacent IgE molecules fixed to Fc receptors triggers degranulation of mast cells resulting in the production and release of histamine, leukotrienes, prostaglandins, and cytokines.
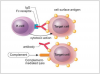
Type II or antibody-dependent cytotoxic reactions are predicated on exposure to an allergen and antigen-specific antibody production dominated by immunoglobulin G (IgG) or M (IgM) isotypes.1,3 Upon re-exposure the antigen binds to the surface of target cells (usually RBCs), the antigen-antibody complexes attract cytotoxic T cells, which release chemical mediators that cause target-cell lysis (Figure 2). Alternatively, antigen-antibody complexes attached to target-cells may activate complement-mediated target-cell lysis.
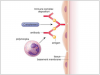
Type III or immune complex-mediated reactions (serum sickness) are predicated on exposure to an allergen and antigen-specific antibody production dominated by immunoglobulin G (IgG) or M (IgM) isotypes.1,3 Upon re-exposure soluble drug molecules form large insoluble antigen-antibody complexes that are deposited in target tissues (e.g., kidneys, joints and lungs) and initiate complement activation, neutrophil and platelet aggregation, and an intense inflammatory response (Figure 3).
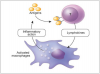
Type IV or delayed T cell-mediated hypersensitivity reactions are predicated on exposure to haptens, which bind to endogenous macromolecules and form hapten-protein complexes.1,3 Langerhans cells phagocytize and process hapten-protein complexes, load them into major histocompatibility complexes, migrate to regional lymph nodes and present them to naïve T-lymphocyte. Upon re-exposure sensitized T cells in target tissues activate macrophages, which mediate direct cellular damage (Figure 4).
Mechanisms of Idiosyncratic ADRs
Idiosyncratic ADRs are observed in a small number of patients. Factors influencing drug response phenotype include age, gender, underlying disease, and genetic and epigenetic mechanisms.1,4,5 Genetic polymorphism and epigenetic factors (i.e., heritable changes in gene function and expression not related to DNA sequencing) influence outcome of pharmacotherapy and have been implicated in both pharmacokinetic and pharmacodynamic variations.
Inherited variations in enzymes that catalyze drug metabolism are the most common causes of variation in response to medications. The effects of genetic polymorphism on drug metabolism are the most prominent with five isoforms of the CYP450 enzymes, i.e., CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Isoenzyme-induced variations in drug metabolism may lead to significant differences in the efficacy and toxicity of drugs.
CYP3A4 is involved in the metabolism of many drugs currently prescribed. Drugs that are substrates of CYP3A4 may competitively inhibit each other. Other drugs may inhibit or induce CYP3A4 activity without being substrates. The consequences of CYP3A4 polymorphism will depend on the intrinsic character of the drug, on the importance of the deficient metabolic pathway, and the existence of alternative metabolic pathways.
Mechanisms of Oncogenic ADRs
Oncogenesis is a complex process involving genetic and epigenetic changes that result in an imbalance between cell division and apoptosis (programmed cell death).1 Among the known factors implicated as potential “initiators” and/or “promoters” of cancer are tobacco, alcohol, solar radiation, ionizing radiation, occupational carcinogens, environmental pollutants, infectious agents, nutrients, and rarely, medications.
Primary oncogenic effects can be produced by drugs (initiators) that damage DNA or by drugs (promoters) that facilitate proliferation of cells carrying precancerous mutations.1 Drugs that act as initiators are drugs that have been converted into reactive metabolites by polymorphic oxidation reactions. These reactive metabolites bind covalently to DNA in proto-oncogenes or tumor-suppressor genes, modify DNA, and lead to mutations.
Proto-oncogenes are normal genes that promote cell division. Oncogenes are proto-oncogenes, which through amplification or mutation became permanently activated allowing abnormal cells to survive and proliferate. Tumor suppressor genes are normal genes that slow down cell division, repair damaged DNA, or initiate apoptosis. Through mutations, tumor suppressor genes are inactivated leading to uncontrolled cell growth.
For example, activated oncogenes and inactivated tumor suppressor genes may alter the expression of cell-cycle regulatory proteins (e.g., cyclin-dependent kinases) that govern the initiation, progression, and completion of cell-cycle events, causing overexpression of cyclins and loss of expression of cyclin-dependent kinase inhibitors. Deregulated cyclin-dependent kinase activity provides malignant cells with a selective growth advantage.
Secondary oncogenic effects are related to therapeutic immunosuppression. Reactivated latent oncogenic viruses can induce mutations, gene amplifications, or chromosome rearrangements in host DNA.6 Human oncogenic DNA viruses include the Epstein-Barr virus, human papilloma viruses, hepatitis B virus, and herpes simplex virus-8; RNA viruses include human T-lymphotropic virus type 1 and hepatitis C virus.
Mechanisms of Teratogenic ADRs
Teratogenesis is the process that results in structural and/or functional defects in a fetus.1,7 A teratogen is a chemical (drug) that can induce teratogenesis. Teratogenicity depends on the ability of a teratogen to diffuse from the maternal circulation across the placenta. The extent of diffusion depends on the chemical nature of the agent (molecular weight, protein binding capacity, lipid solubility, and pKa) and maternal pharmacokinetic factors.
Teratogens present the greatest risk to the embryo during periods of intense mitotic activity. Exposure to a teratogen from the time of conception to day seventeen results in spontaneous abortion. Exposure to a teratogen during organogenesis, i.e., from day 18 to day 55 results in developmental abnormalities (Figure 5). The period from day 55 through the 3rd trimester is a period of fetal growth, exposure to a teratogen affects organ function.
Clinical Manifestations of ADRs
The Council of International Organizations of Medical Sciences (CIOMS) published guidelines on diagnostic criteria and basic requirements for standardized reporting of ADRs.8 This is especially relevant since most reporting is based on single cases. The CIOMS codified ADRs under 21 major headings and defined 179 conditions considered reportable. However, there is no specific major heading for ADRs affecting oral tissues.
ARDs related to immune-mediated and idiosyncratic mechanisms among the 30 most common ADRs associated with therapeutic doses of drugs in the top 200 dispensed by U.S. community pharmacies include rash, pruritus, allergic reactions, urticaria, arthralgia, and anaphylaxis.9 In addition, less common ADRs affecting oral tissues, rare oncogenic and teratogenic ADRs, and some secondary ADRs are discussed.
DailyMed is the official website for FDA-approved label (package insert) information.10 It provides a centralized, standard, comprehensive, up-to-date, look-up-and-download resource for package inserts submitted to the FDA by pharmaceutical companies. The website is user-friendly, it includes strengthened warnings undergoing FDA review, and it is a reliable resource for information on known potential ADRs related to specific drugs.
ADRs Affecting Skin (Mucosa) and Appendages
Rash is listed as the 6th most common ADR associated with the top 200 drugs dispensed by U.S. community pharmacies.9 However, rash is a general term and CIOMS discourages its use as it encompasses virtually all skin eruptions.8 Pruritus or itching may be a symptom of primary skin lesions or less frequently that of a systemic disease.11 However, itching may also be the result of drug-induced histamine release by mast cells unrelated to the immune system or it may reflect a bona fide drug-related allergic reaction.11
Opioid analgesics have a central pruritic action and also have the ability to induce peripheral histamine release.1 Vancomycin and ciprofloxacin can cause red man syndrome characterized by itching followed by the emergence of a “rash” or hives, i.e., urticaria.1 Other drugs that can cause itching and urticaria include NSAIDs, penicillin, and some antifungal agents. These reactions are unrelated to IgE-induced mast cell degranulation and have been called anaphylactoid or pseudoallergic reactions.8
Urticaria is a well-circumscribed erythematous, pruritic plaque on skin associated with the release of histamine and other vasoactive substances from mast cells and basophils resulting in intradermal edema caused by vasodilation.8,11 As noted, this may be due to direct non-allergenic activation of mast cells by drugs or drug-induced cyclooxygenase inhibition-related mast cell degranulation. Chronic urticaria is usually idiopathic or it may be associated with auto-antibodies to IgE receptors causing mast cell degranulation.11
Acute urticaria most often reflects a hypersensitivity or allergic reaction in which allergen-bound IgE initiates mast cell and basophil degranulation (Figure 6 and Figure 7).11 It may be noted in susceptible patients within minutes or hours following exposure usually by contact or inhalation to an allergen such as latex proteins; and it may be precipitated by exposure to many prescription and over-the-counter medications. A common feature of pruritus and urticaria is subcutaneous and submucosal angioedema of target tissues.8,11
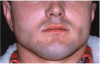
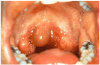
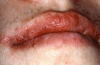
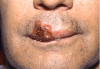
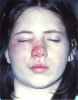
Angioedema may be acute and chronic. Chronic angioedema is rarely IgE-mediated; it is usually idiopathic and may be caused by the chronic ingestion of certain drugs (e.g., penicillin), preservatives, milk, and food additives; and a few cases are hereditary.12 Acute angioedema may reflect a localized IgE-mediated reaction. However, swelling of the extremities, face, lips (Figure 8), tongue, oropharynx (Figure 9), and larynx along with stridor, wheezing, and hypotension are harbingers of systemic anaphylaxis.8,12
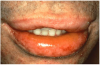
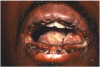
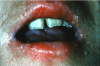
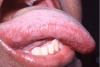
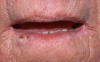
Mucocutaneous ADRs of interest to oral healthcare providers include erythema multiforme (EM). EM is an acute T cell-mediated cytolytic reaction usually to the herpes simplex virus. However, in may be precipitated by NSAIDs, penicillins, anticonvulsants, and sulfonamides. Cutaneous lesions begin as erythematous papules that progress to form the more characteristic iris or target lesions (Figure 10).13-16 Hemorrhagic crusting of the lips (Figure 11) and vesiculoerosive lesions on unattached oral mucosal tissues are diagnostic.
Severity of EM varies from mild (EM minor) to moderate (EM major) and to potentially fatal Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).8,13-16 The majority of SJS and TEN is precipitated by drugs. Oral features of SJS and TEN are similar to those associated with EM. The major difference between SJS and TEN is the distribution of dermal lesions. SJS affects <10% of the body surface while TEN affects >30%. Skin involvement of 15 to 30% of the body surface is considered SJS-TEN overlap.
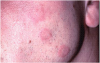
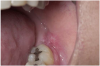
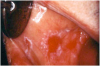
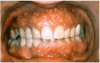
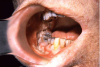
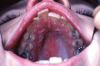
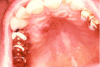
Extrinsic antigenic sources such as drugs have been identified as agents responsible for oral lichen planus (OLP)-like lesions. Drugs such as NSAIDs and ACE inhibitors can act as haptens and alter the antigenicity of epithelial self-antigens. OLP that can be traced to an extrinsic cause is more properly termed a lichenoid reaction.8,13-16 Oral lichenoid lesions most often affect the buccal mucosa (Figure 12 and Figure 13), gingivae, the lateral border of the tongue and may be “reticular,” erythematous, or atrophic.
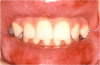
Arthralgia is the 24th most common ADR associated with the top 200 drugs.9 Arthralgia may be described as sharp or dull, stabbing, burning or throbbing, and may range in intensity from mild to severe. Drugs associated with arthralgia include ACE-inhibitors, bisphosphonates, fluoroquinolones, corticosteroids, and vaccines. However, only rarely is arthralgia the result of an adverse reaction to a drug.17 The most common cause of arthralgia is arthritis; other causes include injury and infection.
Hypersensitivity-related ADRs
Type I hypersensitivity reactions or anaphylaxis are acute, IgE-mediated systemic reactions that occur within minutes to hours after parenteral or enteral administration of a drug in a previously sensitized patient.1,3,12 The shorter the reaction time, the more severe the reaction is. Following enteral administration of an allergen the reaction may be delayed or less severe. Anaphylaxis may simultaneously involve multiple organ systems reflected in one or more of the following signs and symptoms:8,12
• Skin: pruritus, erythema, urticaria, angioedema
• Respiratory system: laryngeal edema and/or spasm, bronchospasm
• Gastrointestinal system: abdominal cramps, vomiting, diarrhea
• Central nervous system: anxiety, agitation, loss of consciousness
• Cardiovascular system: tachycardia or bradycardia, hypotension, shock
Type II antibody-dependent hypersensitivity or cytotoxic reactions occur when a drug binds to target cells, usually RBCs, and is recognized by IgG or IgM antibodies.1,3,12 The time required for cytotoxic T cell-mediated target cell lysis or for target cell lysis mediated by the complement system in response to a specific antigen is variable. Clinical manifestations include hemolytic anemia, neutropenia, and thrombocytopenia. Type II reactions are rare, but may be precipitated by several drugs, include penicillin.
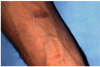
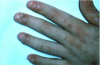
Type III or immune complex–mediated hypersensitivity reactions (serum sickness) may occur within 1-3 weeks after initial exposure to a foreign protein such as monoclonal or polyclonal antibodies prepared from horse, rabbit, or mouse serum.1,3,12 Upon re- exposure, however, serum sickness develops sooner. Reactions clinically similar to serum sickness have also been noted in association with non-protein drugs such as penicillins, metronidazole, tetracyclines (Figure 14 and Figure 15), and NSAIDs.
Common symptoms of serum sickness include fever, cutaneous eruptions (usually urticaria), arthralgia, gastrointestinal complains (nausea, vomiting, diarrhea, or abdominal pain), headaches, myalgia, dyspnea and wheezing, and lymphadenopathy.18 Angioedema of the face and neck may occur. Serum sickness is typically self-limiting although occasional complications such as vasculitis, acute renal failure, glomerulonephritis, and myocardial and pericardial inflammation (chest pain) may lead to morbidity or mortality.18
Type IV hypersensitivity or delayed T cell-mediated reactions are mediated by immunologically committed T lymphocytes. In susceptible patients, cytokines and other mediators of inflammation are released within 2 to 7 days of re-exposure to an allergen.1,3,12 Clinical manifestations include allergic contact dermatitis/mucositis (Figure 16 and Figure 17) or a drug-induced maculopapular rash. With repeated antigenic challenge, the response becomes more profound and includes fever, malaise, and angioedema in target tissues.
Idiosyncratic ADRs
As noted earlier, idiosyncrasy is an unusual reaction of any intensity observed in a small number of the individuals.1,4 When a drug produces its usual effect on a person at an unexpectedly high dose, the patient is said to be hyporeactive. When a drug produces its effect at an unexpectedly low dosage, the patient is said to be hyperreactive. Some of this diversity in response rates can be attributed to differences in the rate of drug metabolism by the various CYP450 enzymes. For example, codeine, a pro-drug, is metabolized by demethylation into morphine, its active metabolite, by the CYP450 isoenzyme 2D6.19 Isoenzyme 2D6 is subject to genetic polymorphism. Up to 10% of patients are poor metabolizers of codeine and do not experience analgesia in response to treatment with codeine. Another 10% of the patients are rapid metabolizers of codeine, i.e., they rapidly convert codeine to morphine and may experience severe toxicity (including death), even with therapeutic doses.
Primary Oncogenesis-related ADRs
In general, drugs that cause direct damage to DNA and/or that interfere with DNA repair are avoided in clinical practice. However, DNA damage and/or interference with DNA repair is the specific intent of cytotoxic alkylating agents used to treat neoplasia and such drugs can cause acute myelocytic leukemia.1 Tamoxifen, an estrogen receptor antagonist used to treat estrogen-sensitive breast cancer, acting as a partial agonist at estrogen receptors in the uterus can cause endometrial carcinoma.1
Teratogenesis-related ADRs
Only a small number of drugs in clinical use have been positively implicated in teratogenesis. Of note for oral healthcare providers are the tetracyclines. They induce enamel hypoplasia, discoloration of teeth, and diminished growth of long bones.20,21 Furthermore, they produce higher rates of neuronal-tube defect, cleft palate, and multiple congenital abnormalities such as neuronal-tube defect with cardiovascular malformation.22
Recent evidence also suggests that acetaminophen (APAP) can act as a hormone disrupter such that it interferes with reproductive and thyroid hormone functions essential for normal brain development.23 Increased risk of attention-deficit/hyperactivity disorder (ADHD)-like behavioral problems or hyperkinetic disorders (HKDs) has been reported among children born to women with a history of frequent APAP use during pregnancy.23
Secondary ADRs Related to Therapeutic Immunosuppression
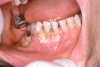
Bacterial infections often contribute to morbidity and mortality associated with therapeutic immunosuppression.14,16 A wide range of bacteria, including odontopathic, peri-odontopathic, and transient pathogens of the oral flora may manifest as ulcerative lesions. The normal signs of infection such as inflammation are not always obvious, with pain, fever, and the presence of a lesion observed most consistently (Figure 18).
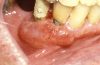
Therapeutic immunosuppression is often complicated by fungal infections such as those associated with the Candidasp.14,16 Oral candidiasis may appear as white, raised, or cottage cheese-like growths that can be scraped off, leaving a red and sometimes hemorrhagic base. Via deglutition, droplet aspiration, or the hematological route oral candidiasis may spread to the esophagus or lungs; and, eventually, may affect all organ systems (Figure 19 and Figure 20).
Viral infections in patients undergoing therapeutic immunosuppression include recurrent herpes simplex virus (HSV) infections affecting the lips and intraoral tissues (Figure 21).16 The ulcerations are quite painful. The optimal period of observation for the detection of recurrent HSV infections is during the 7- to 14-day period following immunosuppression. Primary HSV infections account for less than 2% of infections in this patient population.
Herpes zoster (HZ) is a common clinical manifestation of immunosuppression-induced reactivation of the latent varicella zoster virus.16 It is a localized, painful vesicular rash with an erythematous base restricted to skin and mucosal tissues and follows the distribution of a branch of the trigeminal nerve (Figure 22 and Figure 23). Lesions are usually unilateral, deeper seated and more closely aggregated than those associated with chickenpox.
Epstein-Barr virus (EBV) infection has been associated with a wide range of syndromes in solid organ transplant recipients. In the oral cavity, the EBV has been causally related to hairy leukoplakia, characteristically found on the lateral borders of the tongue in patients with therapeutic immunosuppression (Figure 24). As noted earlier, the EBV is an oncogenic virus, which when reactivated may also cause secondary malignancies.
Secondary malignancies related to therapeutic immunosuppression in susceptible individuals include de novo squamous cell carcinoma of the skin and lip (Figure 25).16 Secondary malignancies related to immunosuppression-induced reactivation of oncogenic viruses include Kaposi sarcoma (Figure 26), lymphoproliferative diseases (Figure 27), Hodgkin's and non-Hodgkin's lymphomas, and spindle-cell sarcoma (Figure 28).16
Withdrawal Syndrome
Withdrawal syndrome is a substance-specific ADR associated with cessation or rapid reduction in the amount of a substance that an individual (usually a substance-dependent person) has been taking for a prolonged period of time and/or in high doses.8 It can occur with alcohol, tobacco, cocaine, amphetamines, and heroin; and following the long-term use of therapeutic agents such as opioids and benzodiazepines. The signs and symptoms of withdrawal of a drug are opposite to the effects of the acute administration of that drug.
Preventing ADRs
Preventing ADRs is a critical part of clinical practice. Oral healthcare providers must have an awareness of and have access to information related to ADRs. To minimize such events, they must develop a rational approach to the use of pharmacotherapeutic agents in the management of oral/odontogenic problems; especially, since in the treatment of most such conditions non-pharmacological intervention such as primary dental care is a more effective and safer alternative than pharmacotherapy.
However, when pharmacotherapy is indicated practitioners must avoid “rationalized activism”. The rational activist assumes that it is better to over-treat than not to treat at all. Even if the risk is considered small, prescribing a drug with the potential to cause an ADR may not be justified. Practitioners must also avoid “reflex prescribing”. The reflex prescriber, the agent of brief encounters, is typically concerned with the patient's symptoms or caters to the patient's expectations.
Benefits should always outweigh the risks when a drug is prescribed. If clinicians were to observe this basic principle routinely, then the number of unnecessary or inappropriate prescriptions would be reduced. Drug therapy should be individualized by taking into consideration both drug- and patient-related variables. Errors in medications, which may lead to ADRs, are related to such factors as progressing age, multiple illnesses, living alone, and poor coping ability of ambulatory patients with their environment.
In addition to the choice and dosage of a drug consider the route of administration and other drugs the patient may be taking. Take time to explain the role of drugs in the treatment of the patient's condition. Pay special attention to impaired intellect, poor vision, and diminished hearing. Simple and clear oral instructions on how and when to take a drug should be given and reinforced by clear labeling and written instructions. Special labels are available for blind or poorly sighted patients.
Assess the patient's response to drug therapy frequently to confirm efficacy and compliance. When new signs and symptoms are reported, rule out ADRs. Adjust dosages and/or discontinue unnecessary medications. Complex regimens and frequent dosing lend themselves to noncompliance. A byproduct of poor compliance is hoarding of drugs, which can further contribute to noncompliance and ADRs as patients may confuse new bottles with old ones or use hoarded drugs for the wrong purpose.
Diagnosing ADRs
The diagnosis of ADRs is highly subjective and imprecise. Complaints such as fatigue, inability to concentrate, and excessive sleepiness have been reported by healthy individuals not taking medications. It is also well known that patients receiving a placebo report ADRs. However, drugs as disease and symptom producing agents should always be considered in the formulation of a differential diagnosis and the following step-wise process can be helpful in assessing possible drug-related adverse reactions:
• Step 1 – Identify the drug(s) taken by the patient.
• Step 2 – Verify that the onset of signs and symptoms was after the initiation of pharmacological intervention.
• Step 3 – Determine the time-interval between the initiation of drug therapy and the onset of signs and symptoms.
• Step 4 – Stop drug therapy and monitor signs and symptoms.
• Step 5 – In rare instances it may be appropriate to restart drug therapy and monitor for recurrence of signs and symptoms.
Reporting ADRs
The FDA has the responsibility for ensuring the safety of all marketed drugs; and, consequently, for maintaining a post-marketing surveillance program to identify ADRs. The success of this program depends on active participation by all clinicians. The FDA launched MedWatch, an initiative designed to educate health care professional about the critical importance of being aware of, monitoring for, and reporting ADRs.24
It is of import to note that the reporting clinician is not responsible for proving causality; a suspected association constitutes sufficient reason to report. The FDA holds the identity of the patient in strict confidence. However, unless otherwise indicated on the reporting form, the reporting clinician's identity may be shared with the manufacturer of the drug. Reports may be sent directly to the FDA by several different mechanisms:
○ Call 1-800-FDA-1088 to report by telephone
○ Download form or call 1-800-332-1088 to request a reporting form
- Submit the form by fax to 1-800-FDA-0178
Based on these reports, the FDA may send out “Dear Health Professional” letters; require warning labels and/or changes to the packaging information (package insert); request further epidemiological investigations and/or manufacturer-sponsored post-marketing studies; and conduct inspections of manufacturers' facilities and/or records. Ultimately, the FDA may require withdrawal of the drug from the market.
Summary
Prerequisites to considering ADRs in the differential diagnosis of a patient's problem include awareness that there are no “absolutely safe” biologically active agent and familiarity with relevant literature about ADRs. It is equally important to recognize that some ADRs occur rarely and detection based on clinical experience or reports in the literature at times is impossible. The timely reporting of ADRs can save lives, reduce morbidity, and decrease the cost of healthcare.
References
1. Conner MW, Dorian-Conner C, Vaidya VS, et al. Drug toxicity - Principles of pharmacology: The pathophysiologic basis of drug therapy, 4th edition. David E. Golan (Ed). Philadelphia, PA. Wolters Kluwer. 2017. 70-86.
2. Williams KA, Taifour ML. Adverse Drug Reactions - Part I. dentalcare.com. Accessed July 24, 2017.
3. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003 Nov 1;68(9):1781-90.
4. Belle DJ, Singh H. Genetic factors in drug metabolism. Am Fam Physician. 2008 Jun 1;77(11):1553-60.
5. Dahlin A, Tantisira K. Pharmacogenomics - Principles of pharmacology: The pathophysiologic basis of drug therapy, 4th edition. David E. Golan (Ed). Philadelphia, PA. Wolters Kluwer. 2017. 87-95.
6. Sevik M. Oncogenic viruses and mechanisms of oncogenesis. Turk J Vet Anim Sci 2012;36(4):323- 329. Accessed July 24, 2017.
7. Chung W. Teratogens and their effects. Accessed July 24, 2017.
8. Council for International Organizations for Medical Sciences. Reporting adverse drug reactions. Definitions of terms and criteria for their use. Geneva. CIOMS. 2000.
9. Roswarski M, Villa KR, Kiersma ME, et al. Prevalence of Adverse Drug Effects/Adverse Drug Reactions in 200 Most Commonly Prescribed Drugs Corrected for Prescription Volume. Pharmacy Practice Faculty Presentations. Las Vegas, NV. 2009. Accessed July 24, 2017.
10. National Institute of Health. U.S. National Library of Medicine. DailyMed. Accessed July 24, 2017.
11. MacNeal RJ. Approach to the dermatologic patient – The Merck manual of diagnosis and therapy, 19th edition. Robert S Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 632-645.
12. Delves PJ. Allergic and other hypersensitivity reactions – The Merck manual of diagnosis and therapy, 19th edition. Robert S Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 1108-1125.
13. Rehmus WE. Hypersensitivity and inflammatory disorders. – The Merck manual of diagnosis and therapy, 19th edition. Robert S Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 683-691.
14. Jacobsen PL, Chávez EM. Clinical management of the dental patient taking multiple drugs. J Contemp Dent Pract. 2005 Nov 15;6(4):144-51.
15. Torpet LA, Kragelund C, Reibel J, et al. Oral adverse drug reactions to cardiovascular drugs. Crit Rev Oral Biol Med. 2004 Jan 1;15(1):28-46.
16. Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004 Jul 1;15(4):221-39.
17. Berman JR, Paget SA. Approach to the patient with joint disease reactions – The Merck manual of diagnosis and therapy, 19th edition. Robert S Porter (Ed). Whitehouse station, NJ. Merck Sharp & Dohme Corp., 2011. 283-297.
18. Alissa HM, Diamond HS. Serum sickness. Accessed July 24, 2017.
19. Gasche Y, Daali Y, Fathi M, et al. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med. 2004 Dec 30;351(27):2827-31.
20. US National Library of Medicine. DailyMed. Minocin - minocycline hydrochloride capsule, coated pellets. Triax Pharmaceuticals, LLC. Updated 12/13. Accessed July 24, 2017.
21. US National Library of Medicine. DailyMed. Doxycycline hyclate capsules - doxycycline hyclate capsule. Hikma Pharmaceutical. Updated 9/09. Accessed July 24, 2017.
22. Czeizel AE, Rockenbauer M. A population-based case-control teratologic study of oral oxytetracycline treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2000 Jan;88(1):27-33.
23. Liew Z, Ritz B, Rebordosa C, et al. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014 Apr;168(4):313-20. doi: 10.1001/ jamapediatrics.2013.4914.
24. U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Safety. MedWatch. The FDA Safety Information and Adverse Event Reporting Program. Reporting Serious Problems to FDA. Instructions for Completing Form FDA 3500. Accessed July 24, 2017.
About the Authors
Kristin A. Williams, DDS, MPH
Dr. Kristen A. Williams is an Assistant Professor, Department of Community Dentistry, and Assistant Dean for Admissions and Student Affairs at Case Western Reserve University, School of Dental Medicine in Cleveland, OH. Dr. Williams received her D.D.S. in 1989, completed a residency in Dental Public Health, and obtained a Master of Public Health in 2005, all at the School of Dental Medicine, Case Western Reserve University. Dr. Williams holds several positions in local professional societies, serves as a reviewer for national public health publications, published extensively in peer-reviewed journals, and presented many scientific programs at local, state and national professional meetings.
Email: kaw14@case.edu
M. Louay Taifour, BDS, DMD
Dr. Taifour earned his dental degree from Beirut Arab University School of Dental Medicine, then completed an AEGD residency and a Restorative Fellowship at Case Western Reserve School of Dental Medicine. Dr. Taifour teaches several pre-doctoral courses and is a full time clinical attending for the AEGD residency program.