You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
In the first part of this series (Compendium, January 2020, compendiumce.com/go/2001), etiological factors associated with thin alveolar bone and tissue, which often leads to dehiscences and fenestrations, were discussed as they relate to predictability of generating buccal bone during surgically facilitated orthodontic treatment (SFOT).1 This second article reviews the surgical and biomaterial factors related to increased predictability of bone augmentation when performing SFOT. As emphasized in the first article, key etiological factors should be diagnosed and addressed prior to performance of combined surgical-orthodontic treatment. Based on the authors' experience and review of the literature, the surgical factors that are outlined and discussed herein critically impact the regenerative outcome of SFOT. These variables and their influence on outcomes are described in the following sections.
Surgery-Related Factors
Flap and Incision Design and Closure
The incision and flap design in SFOT are dictated by several factors associated with the goal and extent of orthodontic/surgical treatment. Where predictability of augmenting the buccal plate is concerned, flap design is one of the most important factors that can impact outcomes.
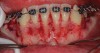
As in any guided bone regeneration (GBR) procedure, soft-tissue management is critical, especially in the anterior mandible because of anatomical factors mentioned in the first article of this series.1 One technique employs a full-thickness sulcular incision, with vertical incisions on each end, allowing for sufficient release and access to enable both periodontal defects and apical corticotomies to be addressed. In addition, primary tension-free closure can be achieved easily using the superficial-layer split-thickness flap technique.2 If sufficient access and flap release can be obtained, an envelope flap without any vertical incisions also can be utilized (Figure 1). Note that the closure of incisions in this technique is different than in the classic GBR procedure3 due to the presence of papillae and root surface in between the facial and lingual flaps. A superficial split is made horizontally through the periosteum and into the submucosal tissue from one end of the flap to the other. This technique allows for easy suturing and avoidance of the forming of any sloughing or clefts throughout the course of healing due to good adaptation of flap margins.
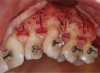
Another common incision design used in SFOT is the papilla-sparing incision in which the papillae are not reflected and a straight horizontal incision is made level with the mid-facial margin (Figure 2).4 The objectives of this flap design are to provide access to the bone for corticotomies and to preserve the height and volume of interproximal tissues.5 This conservative approach avoids any future loss of interproximal tissue and allows for easy access in the presence of orthodontic brackets. Its main disadvantage, besides limited surgical access to bone defects and limited severance of circular periodontal ligament (PDL) fibers involved in postoperative rebound, is compromised closure whereby the connective tissue margins of the flap may overlap the epithelium of non-elevated papillae. This shortcoming can be critical to the final outcome, because it may result in flap dehiscence, further gingival recession, and loss of biomaterial due to exposure. If this technique is used, thorough de-epithelialization of interproximal tissue is recommended to achieve root coverage and primary secure closure. To better allow for tension-free primary closure, the use of vertical incisions and extending the flap past the apical corticotomies are recommended.
Another technique known as piezocision has been advocated as a minimally invasive alternative. It involves the use of a piezosurgery unit to create corticotomies, and it avoids the reflection of a soft-tissue flap.6 This technique, however, has some disadvantages, including the lack of visualization necessary for creating precise corticotomies and limited tissue release, which may affect predictability of bone augmentation.6 In addition, the piezocision procedure may lead to an increase in iatrogenic root resorption when used with orthodontic forces.7
Depth and Location of Corticotomies
Alveolar corticotomies are surgical interventions that are limited to the cortical portion of the alveolar bone. The corticotomy incision pierces one cortical layer while also minimally penetrating the underlying bone marrow.
Wang et al demonstrated in rats that tooth sites subjected to corticotomy procedures revealed significantly more osteoclastic activity at 3 days post-treatment compared with untreated sites.8 In the corticotomy group, the alveolar bone surrounding the dental roots was replaced with multicellular tissue at 21 days. At that time, it was noted that increased concentration of transforming growth factor beta-1, vascular endothelial growth factor, and osteocalcin was found at the mesial border of bone in the corticotomy, whereas a diffuse pattern was observed in the non-corticotomy group.8 Similar changes were noted by Baloul et al.9 This evidence supports the fact that corticotomies and associated tooth movement not only affect the bone remodeling to allow faster tooth movement but also promote subsequent enhanced bone regeneration around those sites.
To the best of the authors' knowledge there are no published guidelines or studies suggesting an ideal depth or location for corticotomy bone cuts. Wilcko et al suggested corticotomy placement exclusively in alveolar bone, avoiding engaging PDL space or cementum.5,10 Their typical design includes vertical incisions between tooth roots, connected apically with a horizontal bone incision.5 Wherever possible, small micro-perforations using a small round bur, for example a #1 round bur, are placed in the adjacent alveolar bone. They further suggested including similar bone incisions on the lingual side of the tooth.5 This was predicated on their understanding that the regional acceleratory phenomenon (RAP), which is a bone remodeling phase that leads to osseous demineralization surrounding the area of cortication, extends for a radius of approximately 8 mm.5 They further suggested that to achieve complete osteopenic changes around the tooth intending to be moved, both buccal and lingual corticotomies are indicated.5
Cohen and Buschang et al demonstrated a relationship between the intensity of the RAP effect and the depth of corticotomy in the area. They found that the greater the depth of the corticotomy into medullary bone, the more profound the RAP effect impacting bone remodeling and tooth movement with accompanying stimulation of growth factors.11
Thus, it is important for both the surgeon and the orthodontist to accurately review the extent of the intended tooth movement; this, in turn, may help the surgeon decide on the depth and location of the appropriate corticotomies. In addition, the corticotomies must extend into the medullary bone to allow for increased blood supply to the grafted sites, thus enhancing graft turnover and new bone formation.
Rotary Versus Piezo Surgical Trauma
In 2004, Robiony et al introduced a new osseous technique involving a piezoelectric surgery device to create osteotomies as an alternative to using rotary instruments.12 One of the main advantages of using a piezosurgical unit is that it works only on mineralized tissues, sparing soft tissues and their blood supply. In addition, compared with rotary instruments the cuts are much finer and less traumatic to osteocytes resulting in more favorable osseous repair and remodeling.13-15
A split-mouth animal study evaluated corticotomy-facilitated orthodontics using piezosurgery instrumentation versus rotary instruments with regard to speed of tooth movement.16 The results showed that tooth movement was 1.6 times faster on the rotary instrumentation side compared to the side using the piezosurgery device. The researchers postulated that rotary instruments induced more trauma to the bone, aggravating the tissue reaction and leading to faster orthodontic tooth movement.16 It has also been shown that RAP-induced orthodontic tooth movement is associated with more active and extensive bone remodeling.17
Although faster tooth movement resulting in increased stimulation of cytokine and growth factor secretion may indirectly impact bone regeneration, it is unclear if this favorable effect surpasses the deleterious influence rotary burs have on osseous vitality, repair, and remodeling compared with piezoelectric surgery.14,18
Amount of Augmentation Required
Little scientific evidence currently exists to definitively address the topic of how much augmentation is required when performing SFOT, and much research is needed in this regard. Nonetheless, it is the authors' opinion that the amount of augmentation needed is dependent on the extent and direction of the anticipated tooth movement. Thus, an interdisciplinary approach should include thorough discussion with the orthodontist to determine the amount and direction of expected tooth movement.
Factors involved in determining the extent of bone augmentation include: resolution of cone-beam computed tomography (CBCT) scans in regard to detecting buccal bone thickness, potential loss of grafting material at coronal parts due to tension and suturing, future resorption of graft material due to lack of blood supply in the dehisced area, host response, and amount of tipping tooth movement utilized.
From observation of GBR procedures, clinicians have learned that during the course of healing, resorption of original graft material placed during the procedure can be expected.19 A review of GBR procedures showed that approximately 33.8% of original graft material was resorbed after 6 months.19 This same consequence is likely applicable to SFOT; thus, over-grafting the buccal plate may compensate for future resorption and graft displacement and allow for greater postoperative tissue thickness and stability. Although some displacement of graft material may occur such that more new bone generation may be noted at the apical portion than elsewhere, tipping teeth facially in the anterior mandible without augmentation can result in greater bone and tissue loss compared to performing simultaneous augmentation.20
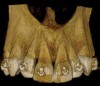
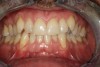
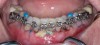
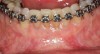
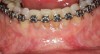
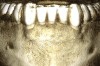
Figure 3 through Figure 8 illustrate an SFOT case where expansion and decrowding were achieved. Although increased osseous thickness was gained mostly at the apical portion of the teeth, these images clearly demonstrate that stable and robust gingival margins were established post-orthodontic treatment. This case also shows that the simultaneous use of grafting allows prevention of tissue loss that typically occurs with significant decrowding and expansion.20
Postoperative Protocol
Short-term post-treatment protocols include use of prophylactic antibiotic therapy, nonsteroidal plus acetaminophen analgesic medications, and a chlorhexidine oral rinse for 2 weeks. There is debate over the possible negative effect of using a nonsteroidal anti-inflammatory drug (NSAID) post-SFOT. Adverse effects of NSAIDs on bone healing in general and on osteoblast activity in particular have been reported and include modulating the growth, differentiation, maturation, and adhesion of osteoblasts, which are essential cell activities associated with bone healing.21-23
An inhibition of wound healing processes and associated inflammation that could be deleterious in bone remodeling, which is important to note especially for patients on long-term NSAID use, has also been suggested.21-23 The present authors, however, conclude that, in correlation with their clinical experience, short-term usage of NSAIDs will not have such impact on bone remodeling and formation. Wheatley et al determined that short-term or low-dose NSAID use did not affect union rates in orthopedic fractures.24 In addition, clinicians should consider that some NSAIDs, such as ibuprofen, have been shown to have fewer adverse effects on bone metabolism compared to other NSAIDs.21
Postoperative Complications
While there appears to be limited data available relative to complications noted in patients treated with SFOT, in the authors' experience potential complications include postoperative infection, sloughing of the soft tissue leading to exposure, and exfoliation of the bone graft material. (Below, in the "Use and Type of Membrane" section, an SFOT case depicts the use of acellular dermal matrix and subsequent sloughing of the soft tissue postoperatively.) With appropriate management, however, most areas will heal uneventfully.
In the case of postoperative infection, it is recommended that it be managed in a similar protocol established for membrane exposure and infection around GBR sites.25 More specifically, if there is clear evidence of infection, such as purulence and swelling around the biomaterial used, irrigation and removal of the infected material are indicated in addition to systemic antibiotic therapy.26 Regarding the sloughing of soft tissue or exposure of biomaterial, the consequence is gingival recession/reduced bone thickness in the area where the tissue loss occurred. No intervention currently is indicated other than palliative treatment. Once orthodontic therapy is complete, the recession can be addressed with the use of an autogenous connective tissue graft; however, facial bone thickness will still be compromised. As for root resorption as a post-orthodontic sequelae, although it can occur after an SFOT procedure, the likelihood of this is actually lower than with traditional orthodontic therapy.17,27
Biomaterial-Related Factors
Type of Bone Grafting Material
The most commonly used biomaterials in augmentation procedures include autogenous bone, demineralized freeze-dried bone allograft (DFDBA), deproteinized bovine bone, or a combination of materials. However, little evidence is found that compares the use of one grafting material versus another in SFOT. In general, since it is placed in a non-contained defect, the biomaterial should have adequate long-term space-maintaining capabilities. Previous reports on SFOT utilized a biomaterial with limited space-maintaining capabilities like DFDBA and showed inconsistent results.28
An alternative biomaterial that may have better space-maintaining capabilities due to its slow resorbing characteristics with delayed graft turnover is deproteinized bovine bone matrix (DBBM). Although this material is slow to resorb, it is still possible to move a tooth into an area of an alveolar ridge that has previously been augmented with DBBM.29 Again, data and research are largely lacking, however histological evidence of new bone formation has been reported.30 Biopsies taken in a private practice 1-year post-SFOT with DBBM have shown the DBBM incorporated into the newly formed bone (Figure 9). DBBM is a slower-resorbing material compared to DFDBA and, therefore, should provide superior longevity of space maintenance, especially when used in non-contained defects. Based on its application and associated outcomes in other augmentation techniques reported previously, it can be concluded that DBBM has better potential to enhance the regenerative outcome of a non-contained alveolar defect compared with other commonly used biomaterials.31
Biologics
Although very little evidence supporting the use of biologics during SFOT can be found in the literature, the same treatment decision strategies and rationale for their incorporation during guided bone and tissue regeneration may be applied to their use in SFOT. The most documented biologics are platelet-derived growth factor-beta (PDGF-BB), enamel matrix derivative (EMD), and autologous blood-derived products, ie, platelet-rich plasma (PRP) and platelet-rich fibrin (PRF).31-36 While conflicting evidence related to autologous products and their use in bone regeneration can be found,37 a recent systematic review by Ghanaati et al supported the use of PRF in bone and tissue regeneration and concluded in its favor.38
According to Ghanaati's review, multiple studies and indications, such as the comparison of socket preservation and ridge augmentation both with and without PRF, were examined, and significant enhancement of new bone formation was reported compared to healing without PRF. Of 101 patients diagnosed with medication-related osteonecrosis of the jaw that were treated with PRF, 96 experienced re-epithelialization and bone regeneration. Regarding periodontal regeneration, PRF alone or its combination with biomaterials significantly improved pocket depth and attachment loss compared to treatment without PRF. More than 70% of the patients were part of studies with a high level of scientific evidence (randomized and controlled prospective studies).38
Because the primary goal of SFOT is to augment and regenerate thin, lost, or missing tissue and bone around teeth undergoing orthodontic tooth movement, the authors believe it would seem logical to apply to SFOT the wound healing principles learned from guided tissue and bone regeneration and its enhancement through the use of biologics. The incorporation of different biologics serving as proliferating and differentiating agents may not only directly affect bone and tissue regeneration but also enhance the overall regenerative outcome via angiogenesis and soft-tissue healing, which are so critical in any wound healing process. When examined in periodontal regeneration, PDGF-BB demonstrated in both animal and human studies the ability to promote bone, cementum, and PDL regeneration in periodontal defects.39,40 Overall, several reports showed greater bone formation, reduced healing times, and enhancement in the regeneration process with PDGF-BB when compared to control groups.33-35 In addition, reports of GBR without the use of a membrane showed enhanced outcomes when PDGF-BB was mixed with a xenograft to augment the alveolar ridge in a minimally invasive regenerative procedure.31,34
With regard to EMD, multiple reports have shown favorable outcomes in periodontal regeneration, resulting in new bone, PDL, and cementum formation.32 In addition, Schwartz and colleagues demonstrated that EMD stimulates proliferation of pre-osteoblasts and differentiation of osteoblast-like cells, as well as proliferation and differentiation of normal osteoblasts.41 With this in mind, incorporating EMD into bone biomaterial in SFOT can potentially further enhance both periodontal and bone regeneration.
Currently, platelet-derived concentrates such as PRF and PRP have been shown to enhance soft-tissue healing, which indirectly can create a better environment for bone growth.31-36,38 In summary, although limited evidence can be found for the utilization of bone biomaterial in combination with biologics for SFOT, given their well-documented enhancement capabilities when used in bone and tissue regeneration, these materials appear to have the potential to improve and enhance the regenerative outcomes of SFOT, particularly in compromised sites with limited blood supply and thin tissue.
Temmerman et al in 2016 showed less bone dehiscence post-extraction around sockets filled with leucocyte- and platelet-rich fibrin (L-PRF) plugs versus control sockets left to heal naturally.36 They reported significant differences for total width reduction between test (-22.84%) and control (-51.92%) sites at 1 mm below crest level. In addition, significant differences were found for socket fill (visible mineralized bone) between test (94.7%) and control (63.3%) sites 3 months post-extraction. The addition of L-PRF is straightforward and can also potentially accelerate wound healing and reduce post-surgical pain to improve overall treatment.42
In light of this information, in SFOT cases where blood supply and tissue thickness are compromised, the incorporation of host-modulating biologics such as PRF or PDGF-BB to improve angiogenesis may help avoid possible complications and enhance the final regenerative outcomes.
Use and Type of Membrane
In general, the use of barrier membranes has been advocated for bone augmentation procedures to achieve both wound stability and space maintenance. It should be noted that when a flap is raised for grafting of the buccal plate, there is no containment of the graft material, which further emphasizes the importance of a membrane. Although extensive evidence has been accumulated regarding SFOT, the evidence related to the use of membranes to cover and contain the graft is limited. Based on clinical experience of treating SFOT using acellular dermal matrix (ADM) membrane, the authors suggest that a membrane is beneficial and recommend that its use be incorporated into SFOT.43
The poor hydrophilicity of ADM may put this type of membrane at risk for postoperative soft-tissue sloughing and graft exposure, resulting in compromised bone thickness and gingival recession (Figure 10 through Figure 13). In addition, long-term outcomes of ADM have shown the membrane to decrease and shrink after several years post-surgery.44,45 In 2004, Harris evaluated a 4-year outcome of dermal matrix and found that the mean degree of root coverage dropped from an initial 93.4% to 65.8% after 4 years.44
Another membrane option is a sugar cross-linked collagen membrane, which is resorbed over a longer period of time than a non-cross-linked collagen membrane. This membrane has been shown to be an effective barrier for 6 months while also promoting the restoration of osseous defects with a bone filler.46
Impact of Planned Tooth Movement
Coscia et al showed that in SFOT cases, although no new bone generation occurred at the coronal third of anterior mandibular teeth, no further deterioration of periodontal support or recession occurred after proclination of the decompensated mandibular incisors.47 Figure 14 and Figure 15 document a case where the mandibular incisors were proclined, but new bone generation still took place at the apical third of the teeth. In cases where proclination is expected, the authors suggest that over-grafting of both soft and hard tissue may be indicated to compensate for the expected dehiscence and loss of thickness. In addition, because graft and wound stability are critical for success, extreme care should be taken with regard to flap release, membrane fixation/stability, and closure.
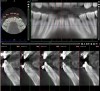
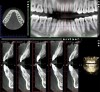
Figure 16 and Figure 17 illustrate a case in which alveolar dehiscences were initially present on the facial aspect of the mandibular anterior teeth (Figure 16). SFOT was performed using ADM as a membrane/soft-tissue thickening agent to cover the deproteinized bovine bone minerals. After the membrane was stabilized via periosteal sutures, a thick alveolar bone was generated (Figure 17). Although some dehiscences remained, the thickness of the bone that was originally present increased substantially. Similar to this case example, Coscia et al emphasized that although the facial bone volume occurs mainly in the mid and apical portions of the root, the final outcome is enhanced in terms of post-treatment tissue thickness and marginal stability.47 However, if orthodontic tooth movement can be modified in terms of its mechanics where proclination of anterior teeth can be avoided, a more robust facial bone can be expected at the conclusion of treatment, as demonstrated in Figure 18 and Figure 19.48 As stated previously, coordination between the surgeon and orthodontist, as it relates to the extent and direction of planned tooth movement, is paramount in deciding the volume and location of augmentation required.49,50
Outcome Measures and Timing
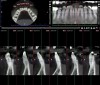
In measuring success of SFOT, CBCT should be the standard means for evaluating the amount and positioning of bone regeneration post-therapy. In general, a scan should be taken no earlier than 6 months post-orthodontic therapy to allow osseous remineralization to occur. Clinically, as mentioned previously, the absence of or minimal gingival recession post-orthodontic treatment could serve as a suitable alternative measure of successful outcome even if bone regeneration was limited.47
Conclusion
SFOT may be an applicable treatment for patients demonstrating crowded dentition and tooth malalignment. A significant aspect of the corticotomy/augmentation approach is related to the accelerated tooth movement associated with the RAP. In a recent systematic review, Haas et al reported an average reduction of almost half the treatment time compared with conventional orthodontics.51
While the accelerated treatment time is a substantial benefit to patients, it is the authors' belief that the primary significance of SFOT is related to greater long-term periodontal support for the teeth and their associated mucogingival complexes in their new positions. This goal of enhancing bone and soft tissue also applies to long-term post-orthodontic tooth stability and reduced risk of future gingival recession often seen with conventional orthodontic treatment.52
In summary, several key surgical factors for improving regenerative outcomes of SFOT have been discussed. These include accurate diagnosis and thorough interdisciplinary treatment planning; suitable flap design, management, and closure; proper selection of biomaterials and their enhancement with biologics when indicated; and postoperative protocol. Additionally, as stated previously,1 primary etiological factors associated with thin alveolar bone must be diagnosed and addressed.
Acknowledgment
The authors acknowledge and thank the orthodontists involved in the treatment of the cases shown in this article: Eladio DeLeon, Jr., DMD, MS; Daniel Levy, DDS, MS; Weston Fortson, DMD; Marielle Beauchamp, DMD; James Gray, DMD; and Steven C. Ricci, DDS, MS.
About the Authors
J. Kobi Stern, DMD, MSc
Associate Professor, Department of Periodontics, Augusta University Dental College of Georgia, Augusta, Georgia
Stuart Beauchamp, DMD
Private Practice specializing in Periodontics, Ormond Beach, Florida
William Baldock, DMD
Periodontal Resident, Augusta University Dental College of Georgia, Augusta, Georgia
Brock J. Pumphrey, DMD
Private Practice specializing in Periodontics, Atlanta, Georgia
Colin S. Richman, DMD
Private Practice specializing in Periodontics, Roswell, Georgia
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Beauchamp S, Richman CS, Baldock W, et al. Factors affecting predicability of buccal bone augmentation in surgically facilitated orthodontic treatment: etiological considerations. Compend Contin Educ Dent. 2020;41(1):18-23.
2. Greenwell H, Vance G, Munninger B, Johnston H. Superficial-layer split-thickness flap for maximal flap release and coronal positioning: a surgical technique. Int J Periodontics Restorative Dent. 2004;24(6):521-527.
3. Simion M, Trisi P, Piattelli A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int J Periodontics Restorative Dent. 1994;14(6):496-511.
4. Greenstein G, Tarnow D. Using papillae-sparing incisions in the esthetic zone to restore form and function. Compend Contin Educ Dent.2014;35(5):315-322.
5. Murphy KG, Wilcko MT, Wilcko WM, Ferguson DJ. Periodontal accelerated osteogenic orthodontics: a description of the surgical technique. J Oral Maxillofac Surg. 2009;67(10):2160-2166.
6. Dibart S, Sebaoun JD, Surmenian J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent.2009;30(6):342-350.
7. Patterson BM, Dalci O, Papadopoulou AK, et al. Effect of piezocision on root resorption associated with orthodontic force: a microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2017;151(1):53-62.
8. Wang L, Lee W, Lei DL, et al. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009;136(6):770.e1-e11.
9. Baloul SS, Gerstenfeld LC, Morgan EF, et al. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139(4 suppl):S83-S101.
10. Wilcko MT, Wilcko WM, Pulver JJ, et al. Accelerated osteogenic orthodontics technique: a 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofac Surg. 2009;67
(10):2149-2159.
11. Cohen G, Campbell PM, Rossouw PE, Buschang PH. Effects of increased surgical trauma on rates of tooth movement and apical root resorption in foxhound dogs. Orthod Craniofac Res. 2010;13(3):179-190.
12. Robiony M, Polini F, Costa F, et al. Piezoelectric bone cutting in multipiece maxillary osteotomies. J Oral Maxillofac Surg. 2004;62
(6):759-761.
13. Gonzalez-Garcia A, Diniz-Freitas M, Somoza-Martin M, Garcia-Garcia A. Piezoelectric and conventional osteotomy in alveolar distraction osteogenesis in a series of 17 patients. Int J Oral Maxillofac Implants.2008;
23(5):891-896.
14. Happe A. Use of a piezoelectric surgical device to harvest bone grafts from the mandibular ramus: report of 40 cases. Int J Periodontics Restorative Dent. 2007;27(3):241-249.
15. Mouraret S, Houschyar KS, Hunter DJ, et al. Cell viability after osteotomy and bone harvesting: comparison of piezoelectric surgery and conventional bur. Int J Oral Maxillofac Surg. 2014;43(8):966-971.
16. Farid KA, Mostafa YA, Kaddah MA, El-Sharaby FA. Corticotomy-facilitated orthodontics using piezosurgery versus rotary instruments: an experimental study. J Int Acad Periodontol. 2014;16(4):103-108.
17. Mostafa YA, Mohamed Salah Fayed M, Mehanni S, et al. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009;136(4):570-577.
18. Haggard CA, Pumphrey BJ, Richman CS, et al. Enhancing periodontal regenerative outcomes with simultaneous orthodontic tooth movement. Compend Contin Educ Dent. 2019;40(1):36-44.
19. Elnayef B, Porta C, Suarez-Lopez Del Amo F, et al. The fate of lateral ridge augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33(3):622-635.
20. Ahn HW, Seo DH, Kim SH, et al. Morphologic evaluation of dentoalveolar structures of mandibular anterior teeth during augmented corticotomy-assisted decompensation. Am J Orthod Dentofacial Orthop. 2016;150(4):659-669.
21. Garcia-Martinez O, De Luna-Bertos E, Ramos-Torrecillas J, et al. Repercussions of NSAIDs drugs on bone tissue: the osteoblast. Life Sci.2015;123:72-77.
22. Chang JK, Li CJ, Liao HJ, et al. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258(2-3):148-156.
23. De Luna-Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ, et al. Effects on growth of human osteoblast-like cells of three nonsteroidal anti-inflammatory drugs: metamizole, dexketoprofen, and ketorolac. Biol Res Nurs. 2015;17(1):62-67.
24. Wheatley BM, Nappo KE, Christensen DL, et al. Effect of NSAIDs on bone healing rates: a meta-analysis. J Am Acad Orthop Surg. 2019;27(7):e330-e336.
25. Lim G, Lin GH, Monje A, et al. Wound healing complications following guided bone regeneration for ridge augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33(1):41-50.
26. Verardi S, Simion M. Management of the exposure of e-PTFE membranes in guided bone regeneration. Pract Proced Aesthet Dent. 2007;19(2):111-117.
27. Liem AML, Hoogeveen EJ, Jansma J, Ren Y. Surgically facilitated experimental movement of teeth: systematic review. Br J Oral Maxillofac Surg. 2015;53(6):491-506.
28. Chackartchi T, Barkana I, Klinger A. Alveolar bone morphology following periodontally accelerated osteogenic orthodontics: a clinical and radiographic analysis. Int J Periodontics Restorative Dent. 2017;37
(2):203-208.
29. Araújo MG, Carmagnola D, Berglundh T, et al. Orthodontic movement in bone defects augmented with Bio-Oss. An experimental study in dogs. J Clin Periodontol. 2001;28(1):73-80.
30. Gray JB, Richman C. Pre-orthodontic periodontal augmentation for lower incisor advancement in adolescent patients. J Clin Orthod. 2018;52
(10):513-527.
31. Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): a new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 2017;37(2):165-173.
32. Mellonig JT. Enamel matrix derivative for periodontal reconstructive surgery: technique and clinical and histologic case report. Int J Periodontics Restorative Dent. 1999;19(1):8-19.
33. Simion M, Nevins M, Rocchietta I, et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009;29(3):245-255.
34. Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007;27(2):109-115.
35. Suarez-Lopez Del Amo F, Monje A, Padial-Molina M, et al. Biologic agents for periodontal regeneration and implant site development. Biomed Res Int. 2015;2015:957518.
36. Temmerman A, Vandessel J, Castro A, et al. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol.2016;43(11):990-999.
37. Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21
(6):1913-1927.
38. Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, et al. Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: how high is the level of scientific evidence? J Oral Implantol. 2018;44(6):471-492.
39. Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213-225.
40. Lynch SE, Williams RC, Polson AM, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16(8):545-548.
41. Schwartz Z, Carnes DL Jr, Pulliam R, et al. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J Periodontol. 2000;71(8):1287-1296.
42. Munoz F, Jiménez C, Espinoza D, et al. Use of leukocyte and platelet-rich fibrin (L-PRF) in periodontally accelerated osteogenic orthodontics (PAOO): clinical effects on edema and pain. J Clin Exp Dent. 2016;8
(2):e119-e124.
43. Ozturan S, Oztunc H, Keles Evlice B. Assessment of the soft tissue volumetric changes following acellular dermal matrix grafts with cone beam computerized tomography. Quintessence Int. 2015;46(2):171-178.
44. Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75(5):734-743.
45. Tavelli L, Barootchi S, Di Gianfilippo R, et al. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12-year follow-up from a randomized clinical trial. J Clin Periodontol. 2019;46(9):937-948.
46. Zubery Y, Goldlust A, Alves A, Nir E. Ossification of a novel cross-linked porcine collagen barrier in guided bone regeneration in dogs. J Periodontol. 2007;78(1):112-121.
47. Coscia G, Coscia V, Peluso V, Addabbo F. Augmented corticotomy combined with accelerated orthodontic forces in class III orthognathic patients: morphologic aspects of the mandibular anterior ridge with cone-beam computed tomography. J Oral Maxillofac Surg.2013;71-(10):
1760.e1-e9.
48. Evans M, Tanna NK, Chung CH. 3D guided comprehensive approach to mucogingival problems in orthodontics. Semin Orthod.2016;22(1):52-63.
49. Mandelaris GA, DeGroot BS, Relle R, et al. Surgically facilitated orthodontic therapy: optimizing dentoalveolar bone and space appropriation for facially prioritized interdisciplinary dentofacial therapy. Compend Contin Educ Dent. 2018;39(3):146-156.
50. Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21(1):9-19.
51. Gil APS, Haas OL Jr, Mendez-Manjon I, et al. Alveolar corticotomies for accelerated orthodontics: a systematic review. J Craniomaxillofac Surg. 2018;46(3):438-445.
52. Makki L, Ferguson DJ, Wilcko MT, et al. Mandibular irregularity index stability following alveolar corticotomy and grafting: a 10-year preliminary study. Angle Orthod. 2015;85(5):743-749.