You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
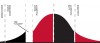
The technology adoption life cycle is a predictable cycle that is observed with emerging technologies in all industries (Figure 1).1 When a technology is first introduced, its users are deemed to be innovators. Next comes a phase in which an early few, known as early adopters, begin to employ the technology, which ultimately leads to the interest of the segment known as the early majority. If the growth rate of the early adopters' segment is too slow, it can lead to a gap or "chasm" in the adoption curve between it and the early majority, making it unclear if the technology will ever become mainstream. And if this chasm isn't crossed, the technology may never become fully utilized.
In dentistry, examples of technology that have demonstrated differing rates of adoption include practice management software, digital radiography, and intraoral scanning. Because the benefits of fully digital implant planning and guided implant placement have become well established during the past 8 years, these technologies have been advancing toward the early majority stage of dentistry's technology adoption lifecycle. As an alternative to guided surgery, robot-assisted implant placement is still in the initial stages of early adoption, but it shows promise regarding its ability to cross the technology adoption chasm into the mainstream.
In the medical setting, robot-assisted surgery emerged during the 1990s, following the emergence of intraoperative navigation at the beginning of the decade. In 2000, robot-assisted surgery gathered significant momentum when the US Food and Drug Administration (FDA) approved the da Vinci surgical system for use in the United States. Today, the use of robot-assisted surgery can be said to have crossed the technology adoption gap for a variety of medical procedures.2-5 Millions of robot-assisted surgeries have been performed in the United States with a high rate of acceptance from the medical community and general population.6 Similarly buoyed by enhanced and augmented accuracy, precision, and intraoperative flexibility, robotic assistance for dental implant surgery may now be poised to leap across the adoption gap.
Guided Surgery
With the use of technologies such as cone-beam computed tomography (CBCT), intraoral scanning, virtual implant planning, and 3D printing, dental implant placement has become more precise and less invasive. When CBCT scan data is merged with intraoral scan data in implant planning software, superficial anatomy (eg, teeth and tissue) can be related to deep anatomy (eg, nerves, sinuses, osseous contours), facilitating restoration-driven implant planning in minutes.7,8 This technology also enables the design and fabrication of surgical guides via 3D printing.9-12 Surgical guides have been increasingly adopted because they have been shown to lead to more accurate and efficient dental implant placement as well as more predictable outcomes.13,14 Guided surgery may increase the probability of success for immediately placed implants, improve clinical outcomes, and enhance the patient's experience because it requires less appointments to complete treatment.15,16 However, even when the data merging and implant planning are performed in an efficient and timely manner, the 3D printing must be completed hours to days in advance of the patient's surgical procedure.17,18 These and other workflow limitations may restrict the adoption of surgical guides to certain procedures and practices.
Dynamic Navigation
Dynamic navigation, also known as camera navigation or intraoperative navigation, was adapted to dentistry from the medical field in the late 1990s to address some of the limitations of static guides.19 Typically, dynamic navigation uses infrared or visible light to track the handpiece and provide real-time information on the position of the drill with respect to a CBCT-based surgical plan.20 The elimination of a fabricated guide enables the surgeon to dynamically respond to intraoperative conditions by changing the plan as needed. With advances in CBCT technology, dynamic navigation has seen increased use in the clinic; however, these systems do not actively prevent their users from moving off plan, and the dental surgeon has to look away from the patient in order to use a monitor for visual navigation.21,22 In addition to other workflow issues, these limitations may impact the eventual mainstream adoption of dynamic navigation.
Robot-Assisted Surgery
Another emerging technology, robotic assistance, was developed to deliver the accuracy and precision of fabricated surgical guides while also providing augmented flexibility during digitally guided dental implant placement.23-26 The first robot-assisted system for dental implant surgery received FDA clearance for use in the United States at the end of 2016,27 and since it became commercially available, the technology has shown promise for crossing the adoption gap from innovators and early adopters into mainstream use.
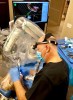
Robot-assisted implant surgery eliminates the fabrication of 3D printed surgical templates, but still provides the benefits of restoration-driven digital implant planning, including linking superficial and deep anatomy to implant position. The proposed digital implant treatment plan may be immediately surgically delivered to the patient. During implant placement, robotic assistance provides physical guidance as well as visual and auditory confirmation, and robotic force feedback limits deviation beyond the prescribed depth and trajectory, leading to the desired implant position and angulation. In this manner, unlike in guided surgery with 3D printed surgical templates, intraoperative modifications may be made when dictated by clinical findings or the surgeon's insight. In addition, robot-assisted procedures improve ergonomics for surgeons because they are performed with haptic guidance and provide monitor-based visual confirmation (Figure 2). To illustrate the promise of robot-assisted implant surgery, the following case reports demonstrate how it can benefit the implant surgeon, the restorative dentist, and most importantly, the patient.
Case Report No. 1
A 64-year-old male patient with controlled hypertension presented to the office for the treatment of tooth No. 12. All that remained of the tooth was a retained root that had been treated with a root canal. It was deemed nonrestorable, and implant reconstruction was advised. Once the patient accepted the proposed treatment, a digital workflow was initiated for the flapless extraction of tooth No. 12 followed by robot-assisted immediate implant placement.
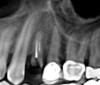
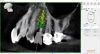
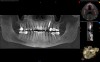
After a CBCT scan (Figure 3) and a digital impression were obtained for diagnosis and treatment planning, the DICOM file from the CBCT scan and the STL file from the digital impression were merged in a treatment planning software application for evaluation. A virtual crown was then created to facilitate a restoration-driven approach. The vital structures and osseous anatomy were identified, and the implant position was planned virtually (Figure 4 and Figure 5).
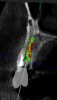
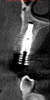
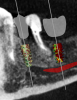
On the day of surgery, a prefabricated tooth-borne splint with an extraoral extension was placed on the patient's contralateral arch and secured with a cold-cure hard relining material. The splint was uniquely designed so that a "breakaway" technique could be used to remove it upon completion of the case. Next, a resin attachment with seven round metal beads, called a fiducial array, was magnetically attached to the splint extension (Figure 6). A new CBCT scan was then taken to capture the fiducial array and relate the splint to the patient's anatomy. After a calibration process was completed to ensure the accuracy of the robotic guidance arm, it was attached to the oral splint and secured. The guidance arm allows the system to identify the patient's anatomy, detect movement, and move with the patient. Profound anesthesia was achieved, and tooth No. 12 was extracted utilizing an atraumatic flapless technique. Next, an implant bur was placed into the robotic handpiece and measured, and accuracy was again confirmed using a predetermined landmark on tooth No. 11. The implant drilling protocol was then completed using the robotic handpiece. Drilling angle, position, and depth are constrained to the plan by the robotic arm, which uses haptic guidance to limit the movement of the handpiece to the proper position by providing physical resistance and feedback during the drilling process. The monitor provides visual navigation and confirmation of the correct angle, position, and depth; however, the surgeon may complete the drilling protocol by viewing either the monitor or the patient directly. After the same robotic protocol was used to place the implant into the osteotomy, allogenic bone was injected and condensed into the osseous defect and a temporary cylinder was placed in preparation for an immediate nonloaded provisional crown. A postoperative CBCT scan confirmed that the dental implant had been placed in accordance with the virtual plan (Figure 7 and Figure 8).
Case Report No. 2
A healthy 60-year-old female presented for an emergency evaluation because she was experiencing pain associated with tooth No. 20. It was mobile, malposed, and given a diagnosis of irreversible pulpitis. In addition, teeth Nos. 19 and 21 had been missing for more than 5 years. After the evaluation, a discussion revealed that the patient desired implant reconstruction of the lower left quadrant. During this initial visit, a CBCT scan was obtained, and the virtual restoration-driven implant planning was completed, confirming that extraction of tooth No. 20 with robot-assisted immediate implant placement at the sites of teeth Nos. 19 and 21 was a viable option (Figure 9 through Figure 13). Splint placement, CBCT capture of the fiducial array, calibration, and landmark confirmation were completed as described in the first case report. Following the administration of intravenous sedation and local anesthesia, tooth No. 20 was atraumatically extracted. Flap access was obtained from the site of tooth No. 18 to the site of tooth No. 22, and robot-assisted surgical implant placement was completed at the sites of teeth Nos. 19 and 21. Intraoperatively, guide pins were placed to evaluate the position of the proposed osteotomies, and it was determined that both implants should be tilted buccally by 0.4 mm. The necessary changes were made in the software application, and the new implant placement plan was immediately ready for robotic implementation. The implants were robotically delivered to their planned positions at the sites of teeth Nos. 19 and 21 and torqued to initial stability at 50 Ncm and 45 Ncm, respectively (Figure 14 and Figure 15). Healing abutments were placed, and the tissue was closed primarily. In this case, robotic assistance facilitated the performance of surgery on the same day that the patient initially presented and permitted intraoperative modifications to be made.
Discussion
Digital implant technology continues to emerge and evolve. Digital implant planning and guided placement have demonstrated the ability to enhance accuracy, efficiency, and predictability.7,13,28-30 Robot-assisted implant placement may provide advances in safety, accuracy, and speed, eliminating the need to fabricate a surgical template prior to surgery as well as facilitating the possibility of same-day implant surgery. Robot-assisted implant placement protocols do require additional time for the placement of the splint, acquisition of the fiducial array, and placement of the robotic guidance arm; however, these aspects can be completed by members of the surgical team to allow for better management of the surgical schedule. Initially, surgeons may experience challenges, such as in adapting to the splint protocol and developing confidence in placement accuracy, but these challenges can be overcome with experience.
Additional motivation for the early adoption of robotics has been generated by the emerging protocols for immediate, full-arch reconstruction and zygomatic implant placement. Currently, bone-supported surgical splints are being developed and tested to achieve these goals. As technology advances, artificial intelligence may even be integrated to assist in the creation of ideal treatment plans. As previously discussed by Frank, before realizing the benefits of digital implant technology, experienced implant surgeons must progress through an evolutionary continuum that begins with denial and then proceeds to exception, acceptance, and finally, adoption.8 In this author's practice, the complete evolutionary process to adopt digital implant dentistry took 15 years. By contrast, the evolutionary process to adopt robot-assisted implant surgery took only 1 year.
Conclusion
In healthcare, technology adoption should be patient focused. Technology that improves the predictability of surgery and patients' outcomes, manages cost, and provides excellent experiences warrants development. Consequently, digital implant dentistry has become mainstream because it enhances the accuracy, efficiency, and predictability of implant treatment. In this regard, the introduction of robot-assisted implant surgery represents a significant development in the evolution of implant dentistry.
About the Authors
Scott Frank, DDS
Oral and Maxillofacial Surgeon
North Shore Smile Surgery
Buffalo Grove, Illinois
Lela Grubisich
Robotics Director/
Surgical Administrator
North Shore Smile Surgery
Buffalo Grove, Illinois
References
1. Moore GA. Crossing the Chasm: Marketing and Selling Technology Products to Mainstream Customers. 1st ed. New York, NY: Harper Business; 1991.
2. Mont MA, Khlopas A, Chughtai M, et al. Value proposition of robotic total knee arthroplasty: what can robotic technology deliver in 2018 and beyond? Expert Rev Med Devices. 2018;15(9):619-630.
3. van der List JP, Chawla H, Pearle AD. Robotic-assisted knee arthroplasty: an overview. Am J Orthop (Belle Mead NJ). 2016;45(4):202-211.
4. Kochanski RB, Lombardi JM, Laratta JL, et al. Image-guided navigation and robotics in spine surgery. Neurosurgery. 2019;84(6):1179-1189.
5. Baek SJ, Kim SH. Robotics in general surgery: an evidence-based review. Asian J Endosc Surg. 2014;7(2):117-123.
6. Smith R. Robotic surgery: the future is already here. LinkedIn website. https://www.linkedin.com/pulse/robotic-surgery-future-already-here-roger-smith/. Published May 2, 2019. Accessed May 6, 2020.
7. Vercruyssen M, Laleman I, Jacobs R, Quirynen M. Computer-supported implant planning and guided surgery: a narrative review. Clin Oral Implants Res. 2015;26(Suppl 11):69-76.
8. Frank S. Evolving with implant dentistry: implement digital technology to enhance accuracy, predictability, and efficiency. Inside Dentistry. 2017;13(8):44-50.
9. Bell CK, Sahl EF, Kim YJ, Rice DD. Accuracy of implants placed with surgical guides: thermoplastic versus 3D printed. Int J Periodontics Restorative Dent. 2018;38(1):113-119.
10. Gjelvold B, Mahmood DJH, Wennerberg A. Accuracy of surgical guides from 2 different desktop 3D printers for computed tomography-guided surgery. J Prosthet Dent. 2019;121(3):498-503.
11. Neumeister A, Schulz L, Glodecki C. Investigations on the accuracy of 3D-printed drill guides for dental implantology. Int J Comput Dent. 2017;20(1):35-51.
12. Reyes A, Turkyilmaz I, Prihoda TJ. Accuracy of surgical guides made from conventional and a combination of digital scanning and rapid prototyping techniques. J Prosthet Dent. 2015;113(4):295-303.
13. Tahmaseb A, Wu V, Wismeijer D, et al. The accuracy of static computer-aided implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):416-435.
14. Choi W, Nguyen BC, Doan A, et al. Freehand versus guided surgery: factors influencing accuracy of dental implant placement. Implant Dent. 2017;26(4):500-509.
15. Berberi AN, Noujeim ZN, Kanj WH, et al. Immediate placement and loading of maxillary single-tooth implants: a 3-year prospective study of marginal bone level. J Contemp Dent Pract. 2014;15(2):202-208.
16. Chen Z, Li J, Sinjab K, et al. Accuracy of flapless immediate implant placement in anterior maxilla using computer-assisted versus freehand surgery: a cadaver study. Clin Oral Implants Res. 2018;29(12):1186-1194.
17. Sigcho López DA, García I, Da Silva Salomao G, Cruz Laganá D. Potential deviation factors affecting stereolithographic surgical guides: a systematic review. Implant Dent. 2019;28(1):68-73.
18. Widmann G, Bale RJ. Accuracy in computer-aided implant surgery--a review. Int J Oral Maxillofac Implants. 2006;21(2):305-313.
19. Watzinger F, Birkfellner W, Wanschitz F, et al. Positioning of dental implants using computer-aided navigation and an optical tracking system: case report and presentation of a new method. J Craniomaxillofac Surg. 1999;27(2):77-81.
20. Mandelaris GA, Stefanelli LV, DeGroot BS. Dynamic navigation for surgical implant placement: overview of technology, key concepts, and a case report. Compend Contin Educ Dent. 2018;39(9):614-621; quiz 622.
21. Block MS. Static and dynamic navigation for dental implant placement. J Oral Maxillofac Surg. 2016;74(2):231-233.
22. Stefanelli LV, DeGroot BS, Lipton DI, Mandelaris GA. Accuracy of a dynamic dental implant navigation system in a private practice. Int J Oral Maxillofac Implants. 2019;34(1):205-213.
23. Grant BTN. Implant surgery with robotic guidance - digital workflows for patient care. Oral Health Group website. https://www.oralhealthgroup.com/features/implant-surgery-with-robotic - guidance-digital-workflows-for-patient-care. Published June 10, 2019. Accessed May 6, 2019.
24. Tillery DE, Rawal S. Robotic-assisted dental implant surgery: a team approach to incorporating Yomi into clinical practice. Lecture presented at: American Association of Oral Maxillofacial Surgeons Annual Meeting. September 21, 2019; Boston, MA.
25. Srivastava R, Jyoti B, Kushwaha S, PK Priyadarshi. Computer aided navigation for predictable dental implantology: a review. Natl J Integrated Res Med. 2019;10(3):63-67.
26. Wu Y, Wang F, Fan S, Chow JK. Robotics in dental implantology. Oral Maxillofac Surg Clin North Am. 2019;31(3):513-518.
27. U.S. Food and Drug Administration. 510(k) premarket notification. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K161399. Updated May 4, 2020. Accessed May 6, 2020.