You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Immediate implant placement into an extraction site was described by Schulte et al in 1978.1 Since then clinicians have utilized advances in technology, instrumentation, materials, and techniques to refine this treatment protocol.2-4 Guided surgery techniques can be used to improve accuracy and precision at healed sites,5 and, more recently, dynamic navigation has further advanced immediate implant placement.6 Combining these technologies and techniques with a minimally invasive approach allows for decreased treatment times and less trauma and postoperative discomfort.7
Anatomical and prosthetic considerations dictate the techniques and materials chosen when performing implant placement.8 To manage predictable patterns of bone resorption, the need for bone replacement or augmentation grafting often arises.9 Prosthetic principles and the use of screw-retained restorations often require immediate implant placement in a more palatal and vertical position with a resultant buccal gap.10,11 Particulate bone grafting of this defect, within existing socket walls or with a partial labial bone defect, enhances bone and soft-tissue stability.12,13 Contour grafting may be indicated to further augment the site for esthetic demands or protect the implant at adjacent bone margins.14
Utilization of dehydrated deepithelialized human amnion/chorion membrane (dHACM) has proven to be an important adjunct when performing minimally invasive grafting for implant procedures.15-17 dHACM naturally possesses important signaling proteins that can facilitate wound healing as well as regulate inflammation and pain.15 The amniotic membrane also contains antimicrobial proteins that are effective against a wide array of species.18,19
In a previous publication (Compendium, March 2019) the authors discussed minimally invasive extraction site management with dHACM with a focus on open socket grafting for alveolar volume maintenance and successful delayed implant treatment.20 Before proceeding with the present article, which will focus on minimally invasive immediate implant placement with dynamic navigation at extraction defects using particulate bone grafting and dHACM, it may be beneficial to review some of the key points of the previous article. Post-extraction site preservation grafting is often indicated to ensure adequate alveolar bone dimensions at pontic sites and effective delayed implant placement to prevent significant loss of ridge volume over time.21 Various graft materials and barrier membranes have been used in such procedures.22 Open-site (non-primary closure) preservation with dense polytetrafluorethylene is an established site preservation technique.23 Upregulation of wound healing has been advocated for medically compromised patients and/or compromised wounds using materials that possess signaling molecules to enhance new tissue formation, as well as to modulate postoperative inflammation and patient discomfort.24 Autologous bone and adjuncts have been investigated for use in site preservation procedures to provide signaling molecules capable of accelerated wound healing.25-27 Among these adjuncts is dHACM, which contains an array of signaling proteins that can expedite wound healing and help control inflammation and pain.15-17 Its use along with a minimally invasive surgical approach reduces these symptoms and enhances open-socket grafting healing. The relatively economical availability of dHACM without the need for harvesting autologous tissues or venipuncture and processing is a significant advance for socket grafting procedures. Patient outcomes with pontic site maintenance and delayed implant reconstruction demonstrated excellent bone and soft-tissue contours.20
Minimally Invasive Immediate Implant Placement
This article will further advance the principles presented in the previous article through the depiction of two case reports. The following cases illustrate the use of minimally invasive immediate implant placement with dynamic navigation at extraction defects utilizing particulate bone grafting and dHACM.
Case 1
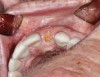
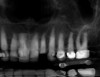
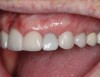
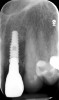
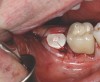
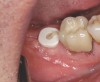
A 70-year-old female patient displayed a fractured root at the maxillary left lateral incisor and was requesting immediate implant reconstruction (Figure 1 and Figure 2). The patient's medical history was significant for appropriate medical management of gastroesophageal reflux disease (GERD), hypothyroid, osteoarthritis, anemia, and history of skin cancer. Clinical evaluation and cone-beam computed tomography (CBCT) assessment (with a dynamic navigation fiducial) facilitated planning with evaluation of labial undercut, buccal bone dimensions, 3-dimensional (3D) implant positioning, and retention screw access path (Figure 3).
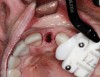
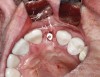
Preoperative chlorhexidine rinses (3 days) and broad-spectrum oral antibiotics were administered. With the patient under intravenous and local anesthesia, minimally invasive root removal and site debridement were completed using repeated aggressive intrabony curettage and irrigation to ensure complete removal of granulations and periodontal ligament remnants. The surrounding bone walls and soft tissue were intact on probing. Site development was completed with dynamic navigation surgery (X-Guide™, X-Nav Technologies, X-navtech.com) (Figure 4 and Figure 5) and immediate implant placement (3.8 mm x 12 mm tapered, BioHorizons, biohorizons.com) against the palatal wall, with good primary stability (implant stability quotient [ISQ] of 72). Limited palatal bone reduction allowed seating of a wide emergence 3-mm tall healing abutment. Buccal gap grafting with mineralized particulate porcine xenograft (ZCore™, Osteogenics Biomedical, osteogenics.com) was placed and covered with a single layer of dHACM allograft (BioXclude®, Snoasis Medical, snoasismedical.com) and secured with inverse "figure 8" polytetrafluorethylene (PTFE) sutures (Figure 6).
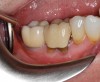
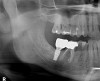
Healing and tissue contours were observed after 10 days (Figure 7) and at subsequent follow-up appointments. Photographs and a radiograph of the restoration at 14 months postoperative demonstrate the successful treatment outcome (Figure 8 through Figure 10).
Case 2
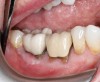
A 68-year-old male patient presented with a failing lower right second molar and retained third molar and was seeking implant restoration at the second molar site (Figure 11 and Figure 12). The patient's medical history was significant for hypertension and cardiac arrhythmia under good control.
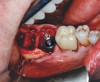
After clinical evaluation, CBCT assessment with a dynamic navigation fiducial was followed by planning for immediate implant placement. This included evaluation of inferior alveolar nerve position, undercut, buccal plate dehiscence, implant positioning in the defect, and retention screw path. The patient was taken to surgery under intravenous and local anesthesia with removal of the mandibular right second and third molar teeth. The sites were aggressively debrided with a mesiobuccal root dehiscence noted, and site development followed with the use of dynamic navigation (Figure 13).
Implant insertion (5.5 mm x 13 mm NobelActive®, Nobel Biocare, nobelbiocare.com) gave excellent primary stability, was level with the residual buccal bone, and achieved an ISQ reading of 74 (Figure 14). Lingual bone contouring (to avoid abutment impingement) was followed with implant gap grafting, which included the mesiobuccal root defect and adjacent extraction site, using mineralized particulate cortico-cancellous allograft (enCore®, Osteogenics Biomedical). A 7 mm x 8 mm poly-ether-ether-ketone (PEEK) healing abutment was inserted and the access opening filled with PTFE tape (Figure 15). A single layer of dHACM (Figure 16) was inserted on the bone graft with tissue forceps and saturated with saline to seal the implant-soft-tissue interface. Suturing with 4-0 chromic gut secured the site, using inverse "figure 8" at the anterior and interrupted sutures at the posterior margin (Figure 17).
Healing of the site was observed on several occasions post-surgery, including at 4.5 months (Figure 18). Excellent gingival and underlying bone contours were revealed in 24-month photographs and panoramic x-ray (Figure 19 through Figure 21).
Discussion
Clinical use of dHACM in minimally invasive dental procedures follows a broad base of medical applications. dHACM is obtained from healthy mothers and infants after elective Caesarian section. Consistent with US Food & Drug Administration (FDA) and American Association of Tissue Banks regulations and guidelines, donated placental tissues are "quarantined" until all tissues, donating mother, and infant are tested and cleared of any "relevant communicable disease agent or disease."28 Current screening protocols test the following: HIV-1 and HIV-2 plus 0 antibody, syphilis (serologic test), hepatitis B surface antigen, HIV type 1 (nucleic acid test [NAT]), hepatitis B core antibody, hepatitis C antibody, human T-cell leukemia virus (HTLV-1 and HTLV-2) antibody, hepatitis C virus (NAT), hepatitis B virus (NAT), and West Nile virus (WNV) (NAT) (WNV NAT screening conducted on donors based on exposure risk per FDA Guidance for Industry).
Cleared tissues are processed through a proprietary method (PURION®, MiMedx, mimedx.com) in which important signaling proteins are preserved, inducing hematopoetic and mesenchymal stem cell recruitment to the membrane.29 dHACM has an initial soluble component of signaling molecules, which elute from the extracellular matrix during the initial days after wound placement, and larger quantities of bioactive proteins are released over weeks as the extracellular matrix is degraded.30 Amnion/chorion displays an immune-privileged status; therefore, dHACM does not produce any host foreign body reaction to the preserved cellular material, cell membrane-associated proteins, and/or intracellular proteins.31 Preservation of anti-inflammatory mediators in the dHACM allows for a diminished postoperative inflammatory response and less postoperative pain, and enables a 5-year shelf life.15,32
Immediate implant placement can significantly reduce time to restoration after tooth extraction. Challenges with positioning and primary stability can be overcome with tapered implant designs, aggressive thread patterns, and planning/placement with dynamic navigation. The two case studies presented showed real-time 3D graphic visualization of drill angulation, depth, and tip position with dynamic navigation surgery. Planning and guidance with dynamic navigation accomplishes accurate, precise site preparation and implant placement with ideal screw-access positioning in the final restoration.6 Prosthetic planning for the use of screw retention typically results in a "palatal" and more "upright" position, with a resultant buccal gap from the implant surface to the internal aspect of the buccal wall.10 Regarding implant size selection, adequate distance to the adjacent teeth or implants should be maintained and result in a buccal gap of 2 mm to 3 mm in the esthetic zone (as in Case 1) and 4 mm to 5 mm at molar sites (as in Case 2). A larger gap dimension at immediate defects does not compromise dimensional stability after healing, but, rather, predictable buccal alveolar contours can be attained with grafting.8 Case 2 further demonstrates management of a mesiobuccal root dehiscence defect12 using particulate grafting of the buccal bone gap and dHACM at the soft-tissue-healing abutment interface. Application of this treatment approach shows ideal bone and soft-tissue regeneration and optimal implant positioning and abutment emergence, thus likely enhancing hygiene and long-term success.
Conclusion
Minimally invasive immediate implant placement is presented at single- and multi-rooted extraction sites using dynamic navigation site preparation and grafting with particulate bone and dHACM. dHACM is a cost-effective adjunct to enhance open wound healing at immediate and grafted sites by reducing pain, providing antibacterial and anti-inflammatory properties, and delivering biologically sustained release of growth factors. Application of these technologies, materials, and techniques can potentially maximize esthetic and functional outcomes for patients with longevity. Immediate implant placement helps reduce treatment time, patient discomfort, and cost for implant restorations. Patients will benefit from continued research and pursuit of these minimally invasive techniques.
About the Authors
Daniel Cullum, DDS
President, Implants Northwest LIVE Learning Center, Coeur d'Alene, Idaho; Visiting Lecturer, University of California Los Angeles and Loma Linda University Departments of Oral and Maxillofacial Surgery; Consultant with Snoasis Medical, Golden Colorado; Private Practice specializing in Oral and Maxillofacial Surgery, Coeur d'Alene, Idaho
Mark Lucas, DDS, MS
Clinical Assistant Professor, University of Colorado School of Dental Medicine, Aurora, Colorado; Vice President of Research & Development, Snoasis Medical, Golden, Colorado
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Schulte W, Kleineikenscheidt H, Lindner K, Schareyka R. The Tübingen immediate implant in clinical studies [in German]. Dtsch Zahnarztl Z. 1978;33(5):348-359.
2. Gelb DA. Immediate implant surgery: three-year retrospective evaluation of 50 consecutive cases. Int J Oral Maxillofac Implants. 1993;8(4):388-399.
3. Benic GI, Mokti M, Chen CJ, et al. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: a clinical and cone beam computed tomography study. Clin Oral Implants Res. 2012;23(5):560-566.
4. Froum S, Casanova L, Byrne S, Cho SC. Risk assessment before extraction for immediate implant placement in the posterior mandible: a computerized tomographic scan study. J Periodontol. 2011;82(3):395-402.
5. Tahmaseb A, Wismeijer D, Coucke W, Derksen W. Computer technology applications in surgical implant dentistry: a systemic review. Int J Oral Maxillofac Implants. 2014;29(suppl):25-42.
6. Block MS, Emery RW, Cullum DR, Sheikh A. Implant placement is more accurate using dynamic navigation. J Oral Maxillofac Surg. 2017;75(7):1377-1386.
7. Cullum D, Deporter D. Immediate implant placement for single- and multi-rooted teeth. In: Cullum D, Deporter D, eds. Minimally Invasive Dental Implant Surgery. Hoboken, NJ: Wiley; 2016:337-366.
8. Ferrus J, Cecchinato D, Pjetursson EB, et al. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin Oral Implants Res. 2010;21(1):22-29.
9. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;
31(10):820-828.
10. Le BT, Borzabadi-Farahani A, Pluemsakunthai W. Is buccolingual angulation of maxillary anterior implants associated with the crestal labial soft tissue thickness? Int J Oral Maxillofac Surg. 2014;43(7):874-878.
11. Chu SJ, Salama MA, Salama H, et al. The dual-zone therapeutic concept of managing immediate implant placement and provisional restoration in anterior extraction sockets. Compend Contin Educ Dent. 2012;33(7):524-534.
12. Le BT, Borzabadi-Farahani A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J Craniomaxillofac Surg. 2014;42(5):552-559.
13. Block MS, Scoggin ZD, Yu Q. Assessment of bone width for implants in the posterior mandible. J Oral Maxillofac Surg. 2015;73(9):1715-1722.
14. Buser D, Chappuis V, Kuchler U, et al. Long-term stability of early implant placement with contour augmentation. J Dent Res. 2013;92(12 suppl):176S-182S.
15. Hassan M, Prakasam S, Bain C, et al. A randomized split-mouth clinical trial on effectiveness of amnion-chorion membranes in alveolar ridge preservation: a clinical, radiologic, and morphometric study. Int J Oral Maxillofac Implants. 2017;32(6):1389-1398.
16. Wallace S, Cobb C. Histological and computed tomography analysis of amnion-chorion membrane in guided bone regeneration socket augmentation. J Implant Adv Clin Dent. 2011;3(6):61-72.
17. Holtzclaw D, Toscano N. BioXclude placental allograft tissue membrane used in combination with bone allograft for site preservation: a case series. J Implant Adv Clin Dent. 2011;3(3):35-50.
18. Zare-Bidaki M, Sadrinia S, Erfani S, et al. Antimicrobial properties of amniotic and chorionic membranes: a comparative study of two human fetal sacs. J Reprod Infertil. 2017;18(2):218-224.
19. Heidarzadeh S, Ghasemian A, Kalafi Z, et al. Antimicrobial effects of amniotic membrane on some bacterial strains. Immunopathol Persa. 2018;4(2):e25-e25. doi:10.15171/ipp.2018.25.
20. Cullum D, Lucas M. Minimally invasive extraction site management with dehydrated amnion/chorion membrane (dHACM): open-socket grafting. Compend Contin Educ Dent. 2019;40(3):178-183.
21. Van der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36(12):1048-1058.
22. Troiano G, Zhurakivska K, Lo Muzio L, et al. Combination of bone graft and resorbable membrane for alveolar ridge preservation: a systematic review, meta-analysis, and trial sequential analysis. J Periodontol. 2017;1-17. doi: 10.1902/jop.2017.170241.
23. Hoffman O, Bartee BK, Beaumont C, et al. Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: a retrospective non-randomized study. J Periodontol. 2008;79(8):1355-1369.
24. Kou X, Xu X, Chen C, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10(432). doi: 10.1126/scitranslmed.aai8524.
25. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
26. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-535.
27. Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perio-implantology: the PRF. Implantodontie. 2001;42:55-62.
28. Part 1271 - Human Cells, Tissues, and Cellular and Tissue-Based Products. Silver Spring, MD: US Food & Drug Administration; April 1, 2018. 21CFR1271.60. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1271.60. Accessed September 24, 2019.
29. Maan ZN, Rennert RC, Koob TJ, et al. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res. 2015;193(2):953-962.
30. Koob TJ, Lim JJ, Massee M, et al. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
31. Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which fetus avoids rejection by the maternal immune system. Reproduction. 2011;141(6):715-724.
32. Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 2010;81(12):1797-1804.