You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Nearly half of all US states mandate identification of dentures with labels including the wearer's name. In recognizing the need to enforce control over critical personal property in dense population settings (such as for personnel of extended residential and hospital care facilities), almost all these measures include minimal requirements for the patient's readable identifier to be fixed to or in the body of the denture whenever possible.
Benefits of identification are obvious when properly labeled lost dentures are recovered at extended care facilities or hospitals, avoiding consequences of lost appliance function, inconvenience, cost, and time involved for prosthesis replacement. Such identification offers great value for cost savings and health preservation. If all removable appliances are labeled in a large residential facility, caregiving staff may efficiently isolate, clean, and store several residents' dentures nightly for reinsertion the next morning. Similarly, a lost labeled removable orthodontic retainer found in a school setting may be returned to service quickly and cost-effectively. The ADA's 2004 publication "The Dentist's Role in Forensic Identification: The Release of Dental Records & Radiographs, and Denture Labeling" includes a resolution that "the American Dental Association urge constituent societies to actively support the use of uniform methods of marking dental prostheses for forensic identification purposes."1
While it is understood that some dentures cannot be labeled due to limitations of space for label placement, material compatibility, or other factors, all laboratories should attempt to add identification to every removable dental appliance as a standard service. In addition to the cost and health benefits mentioned above, there are other compelling reasons for this identification. First, label placement involves minimal investments in cost, time, and labor. Second, with recent advances in removable appliance identification and computerization, contact information for the dental laboratory and/or dentist, as well as other relevant facts, may be immediately linked electronically to appliance labels as an integral and permanent component of that appliance. For example, under proper security protocols, an appliance label can be used for access to detailed information stored electronically on a secure computer database by the laboratory or dental office. This information may include fabrication details of the appliance (ie, tooth shade, tooth mould, etc), pertinent patient information including recommended maintenance schedule intervals for the patient to follow, and any other details as allowed by regulatory statutes. Finally, labeling extends benefits to other healthcare personnel and caregivers in managing and tracking appliances throughout patient treatment or rehabilitation, reducing appliance loss and the need for replacement.
Additionally, labeling helps establish laboratories and/or dental offices as enduring essential referral sources throughout the service life of that appliance and allows continuity of appliance care and maintenance for the patient. Furthermore, integration of laboratory contact information into labeling promotes the readiness to provide needed removable appliance services or repair.
Basic Identification Labeling of Removable Appliances
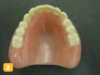
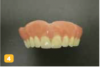
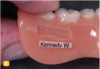
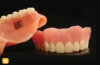
There are several basic methods for removable dental appliance identification. Some technicians suggest that fabricated appliances be photographed before delivery to supplement identification. Photos are stored as a permanent part of the patient record (Figure 1 through Figure 4).
Technicians can easily insert low-cost permanent labels into acrylic dentures and other removable appliances (Figure 5 and Figure 6). An area and depth of acrylic is removed from a posterior flange or other posterior space for the label. Placement should be in a thick material area and acrylic reduction must not compromise appliance strength. The label is fixed into position with clear acrylic and polished flush with the flange or selected area. Note that retentive undercuts in label channels may be required when placing clear acrylic in flexible appliances. The patient's name is now part of the appliance for identification. Information on the label must be compatible with government regulations and applicable industry standards and if possible should be tamper-resistant and/or tamper-evident.
Some identification systems can be purchased as a kit (Figure 6). These kits serve the property identification needs of families, extended care facility managers, hospital administrators, or other care providers to identify dentures or other appliances. Applying identification is done with a supplied marker pen to write the name over a cleaned posterior flange area of the denture, which is then sealed with the provided clear sealant (Figure 7).
Another method is to type the wearer's name on paper with a computer in small bold font (such as 6-point font shown in Figure 8 and Figure 9). The name is carefully cut out. Cutting the label close to the name minimizes its size and eases insertion. Optionally, the label is covered with protective, clear, light-cured composite sealant, clear medical-grade tape, or other suitable material before being embedded in clear acrylic (Figure 8 and Figure 9).
Many types of labels can be placed in the appliance form during stages of final processing at conversion of wax set-ups into acrylic, thus eliminating post-process labeling procedures. Other variations of labeling products are similar in the purpose of identifying the appliance wearer, including the use of nearly invisible label backgrounds (Figure 10) and cost-effective shrink film labels (Figure 11).
Upon appliance delivery, the dentist or denturist should educate the patient on the label's purpose, size, and location to assure acceptance.
Basic Identification with Contact Information
It is possible to present other information as permitted by label space and patient and regulatory authorization. Using a label with name and phone number or email address is possible; the font will be very small but can be read with a magnifier. Label information offers more than notifying the patient on lost appliance recovery. If the phone number is to the attending dental office or laboratory, the caller has an immediate resource and easy reference for appliance evaluation, repair, or maintenance.
Technological Advances: Auto ID/AIDC
Optical barcode and wireless electronic labels are available for insertion into acrylic bodies of finished removable appliances (Figure 12 and Figure 13) and may be placed along with basic written labels as space permits. Information is scanned with properly enabled instruments. Many smartphones are routinely equipped with appropriate hardware for scanning optical and wireless labels, and can scan immediately using an appropriate app. The collection and display of label information by specialized electronic and/or optical instruments in real time is often referred to as automatic identification (Auto ID) or automatic identification and data capture (AIDC). An optical barcode or wireless label that is encoded for scanning by an automated interface device is often referred to as being "machine-readable." When possible, it is preferable to include a written label with the wearer's name in proximity to any machine-readable label placed.
Once embedded, Auto ID/AIDC labels are mostly resistant to the effects of the oral environment. Instructions and methods for label placement may vary. The manufacturer's or vendor's directions must be carefully followed.
Auto ID/AIDC may involve three basic components:
• A machine-readable label that is unique and assigned to only one appliance, and often packaged with a laboratory and/or patient information certificate associated only with that one unique label;
• An automated interface device scanner (or "reader") with sensors to gather information on the label; and/or
• A computer database or secure website with data linked to the label information as scanned by the interface device scanner. The label is assigned only to identify that one specific appliance, and the appliance is scanned outside the mouth within close proximity to the scanner. Authorized and qualified personnel can then access a database file associated with the label, where information on the appliance is stored. Appropriate protective measures for prevention of cross-contamination must be observed when scanning any removable appliance label.
Standardized and proprietary security measures may be incorporated across any one or all of the components mentioned above to protect the validity and integrity of data that is scanned, stored, or transmitted.
Auto ID/AIDC and Labeling of Removable Appliances with Optical Barcode Labels
Optical barcode labels are common in the tracking of retail goods or manufactured products. Barcode labels are simply images comprising special line pictures calibrated to communicate information when scanned by optoelectronic devices, such as found with smartphone cameras. Barcode designs and the information contained on the labels usually conform to international standards (such as GS1) and allow universal interface scanning by many types of optoelectronic devices. Scanning devices can be either instruments dedicated solely to optical barcode scanning or multifunctional devices that are capable of providing many different functions including this scanning, such as smartphones.
Variations of optical barcode labels include quick response (QR) codes, also referred to as "flash codes" and data matrix codes. These codes are usually compact 2-dimensional optical codes that can be scanned by enabled smartphones yet differ slightly in appearance and configuration. Once scanned, a QR or data matrix code label may hold thousands of numeric or alphanumeric characters and/or can be used to activate a direct link to an internet connection and secure website where further information is available for communication. Data matrix codes are preferred for greater reliability when used on smaller labels in identifying removable appliances.
Figure 13 and Figure 14 show an optical data matrix code label for removable appliances. Scanning the barcode on a label or its certificate with an appropriately equipped smartphone at close range (about 1 inch) links that code to a secure website registry for removable appliances (Figure 16). If scanning is not possible, or if limited space on the appliance prevents full label placement, the alphanumeric identifier portion can be excised from the label and placed separately in a smaller profile. Again, specific data regarding that appliance can include details about appliance fabrication, maintenance, specifications of implants associated with the appliance, and other pertinent and appropriate patient information, as well as dentist or physician contact information, etc. Access permission to secure website data is authorized when the identifier is entered at the website login with appropriate passwords, other qualifiers, etc.
Auto ID/AIDC with Machine-Readable Wireless Labels (RFID/NFC)
Wireless radio frequency identification technology has merit in denture identification. Radio frequency identification is now being used much like optical barcodes in the retail and industrial markets. Radio frequency identification (RFID), also known as near field communication (NFC), is a method of encoding and fixing digital information wirelessly onto a miniature silicon memory chip often called a "tag." Like optical barcode labels, RFID/NFC tags and equipment comply with accepted industry standards according to ISO standards (ie, ISO 18000-3,2 13.56 MHz operating frequency, etc) and other regulations to assure uniformity in use and access.
Digital information stored on tags can be repeatedly retrieved at any time using a special radio system called a "reader" that transmits an activating signal to energize the tag (Figure 17). Once energized, the tag responds by broadcasting stored digital information back to the reader. The stored digital information is then converted into readable text on a monitor and/or initiates a secure internet link to information. The reader is said to "interrogate" the tag by activating it, then the information is sent back to the reader. If more than one tag is in range near a reader, special "anti-collision" technologies incorporated into the system allow the reader to present each individual tag separately. Many smartphones that can scan optical barcodes are also capable of reading the RFID/NFC data (Figure 18 and Figure 19).
Care must be taken when placing these tags. It is important to note that RFID/NFC functions may be hindered or blocked if the tags are placed too close to any metallic components, such as cast metal frameworks.
A reader/writer is a self-contained wireless interface device dedicated for transmitting data from (reader) and to (writing) tag data storage (Figure 20). The combination device is powered by USB connection to a computer or by a commonly available external rechargeable battery pack. Optimal working distance between readers, reader/writers, and tags is about one-half inch or less.
Data on NFC/RFID tags can be placed by different methods. For the first option, a fixed permanent identification number is electronically stored on the tag and linked to a secure website or telephone database when the tag is interrogated for its identification number. The identification number is unique, cannot be modified or deleted, and is assigned only to that one specific appliance. While many smartphone systems are enabled as open-format RFID/NFC readers for retrieval of identification numbers, only authorized and qualified personnel can access the secure, government-compliant database file associated with detailed appliance information. Again, database information may include dates and methods of denture fabrication and maintenance; the contact information for the patient, dentist, or physician; and so on. As another option, using the appropriate programming and security protocols, a reader/writer can also be used to wirelessly access, add or edit (ie, write), or transfer the information stored on that tag. Some tags have internal storage capacity for writing as many as seven lines of data-some substantially more-with each line holding 16 alphanumeric characters (Figure 20). Most types of tags have features that allow readers/writers to lock data permanently in place and/or prevent or limit access to protected label data by unauthorized persons.
Oral Appliance Labeling and the Concept of Unique Device Identification (UDI)
The FDA has issued rules and regulations for a unique device identification (UDI) system to register medical and dental devices with specialized descriptive labels (such as in Figure 21). Like the machine-readable labels in dental appliance labeling, the FDA UDI is also linked to the Global Unique Device Identification Database (GUDID) website, which contains key device identification information regarding medical/dental devices with unique device identifiers. Some stored information on GUDID is open for public review.
Under FDA regulation 21 CFR 801.20, a UDI is required on every medical and dental device label and all higher levels of device packaging unless excluded (or, in FDA terms, "excepted") under FDA authorization.4 For the devices that must bear UDIs on their labels, are intended to be used more than once, and are reprocessed between uses, 21 CFR 801.45 requires the devices themselves to be directly marked with a UDI.5 The FDA explains "to be used more than once" as the same device being used by more than one person. Therefore, UDI direct marking would not apply to most restorations, including prostheses fabricated by dental laboratories, since each restoration and/or prosthesis is individualized expressly for a single patient.
For dental laboratories that manufacture devices requiring FDA marketing authorization-referred to as a 510(k)-and that are subject to labeling requirements, the FDA requires each manufactured device to bear a UDI on the device label and higher levels of packaging. Machine-readable labeling presented here can likely be modified and adapted to comply with FDA regulatory requirements for the UDI.
Conclusion
Laboratories should endorse the benefits of removable appliance labeling not just for identification but also to demonstrate and affirm their full and comprehensive participation in prosthetic care. Regardless of what label type the laboratory uses, laboratories have the opportunity to share information associated with their products and remain a ready and reliable source for serving the prosthetic needs of patients and dentists. When paired with methods to access the allowable information and appliance history, labeling is more than a required part of the appliance. It is an assurance that the fabricating laboratory, dentist, and other healthcare members stand ready to participate and contribute in serving the needs and interests of the patient.
Artists label their artwork with their signature to justify their involvement and pride in creating the finished piece. Removable appliances are just as much a viable art form as they are instruments of oral rehabilitation. Labeling them can reveal the source of and details about who created this art.
About the Authors
Gregory S. Jacob, DDS
Private Practice
Glenview, IL
Don L. Powrie
President
DLP Design
Allen, TX
References
1. The Dentist's Role in Forensic Identification: The Release of Dental Records & Radiographs, and Denture Labeling. American Dental Association Web site. http://www.ada.org. Published 2004. Accessed April 25, 2019.
2. Arizona Joins States Requiring IDing Dentures. JDT Unbound. October 2011. http://www.jdtunbound.com/files/pdf-files/JDT_10_11_Web.pdf. Accessed April 5, 2019.
3. ISO/IEC 18000-3:2010(en): Information technology - Radio frequency identification for item management - Part 3: Parameters for air interface communications at 13,56 MHz. International Organization for Standardization Web site. https://www.iso.org/obp/ui/#iso:std:iso-iec:18000:-3:ed-3:v1:en. Published November 2010. Updated 2017. Accessed April 25, 2019.
4. §801.20: Label to bear a unique device identifier (21 CFR 801.20). U.S. Food and Drug Administration Web site. https://www.ecfr.gov/cgi-bin/text-idx?SID=73efb5f1f8aa78a9a7586a241a51b258&mc=true&node=se21.8.801_120&rgn=div8. Published September 24, 2013. Current as of April 23, 2019. Accessed April 25, 2019.
5. §801.45: Devices that must be directly marked with a unique device identifier (21 CFR 801.45). U.S. Food and Drug Administration Web site. https://www.ecfr.gov/cgi-bin/text-idx?SID=73efb5f1f8aa78a9a7586a241a51b258&mc=true&node=se21.8.801_145&rgn=div8. Published September 24, 2013. Current as of April 23, 2019. Accessed April 25, 2019.
Editor's Note: This article has been edited by the publishers.