You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
In September 2012, the US Centers for Disease Control and Prevention (CDC) reported that approximately half of American adults over the age of 30 have periodontal disease, and it is even more prevalent among those aged 65 and older.1 If left untreated, the potential risks for extensive hard- and soft-tissue defects may increase significantly. Periodontal defects resulting from periodontitis can cause significant destruction of the periodontium, which is a complex organ consisting of alveolar bone, periodontal ligament, cementum, and gingiva. Loss of the periodontium may ultimately lead to the loss of natural dentition or its form and function.
The ultimate goal of periodontal therapy is to help patients maintain a healthy, functional, and periodontally stable dentition, which may correlate with overall health. This goal is accomplished by treating the infection, which is caused by pathogenic periodontal biofilm, and arresting or slowing further attachment and bone loss, ultimately preventing tooth loss.2
A significant paradigm shift has occurred during the past several years because major advancements have been made in the treatment of periodontal disease. The periodontium's response to conventional periodontal flap and/or resective surgery has historically been through the process of repair. Repair has been defined as, "the healing of a wound by tissue that does not fully restore the architecture or function of the part." Following conventional flap surgery, epithelial cells of the gingival tissues proliferate and migrate at a faster rate than the cells of the underlying connective tissue, resulting in the formation of a long junctional epithelium.3,4
Another type of healing, regeneration, is described in the American Academy of Periodontology's (AAP) Glossary of Periodontal Terms as, "the reproduction or reconstitution of a lost or injured part." Expanding on this, the concept of periodontal regeneration can be further defined as, "the restoration of lost periodontium, including the formation of new bone, new cementum, and a functionally oriented periodontal ligament." It is because of this reproduction of lost or injured parts that the form and function of these structures can be restored.5 Histological evaluation is the ultimate standard for determining true periodontal regeneration; however, the morbidity associated with these evaluations presents significant challenges. Therefore, histology is typically used to demonstrate the potential of a periodontal regenerative technique, and clinical outcomes, such as probing depths, direct bone measurements, and radiographs, are used to assess the clinical efficacy.6 At the cellular level, periodontal ligament progenitor and bone cells must migrate and attach to the denuded root surface as well as proliferate and mature into an organized and functional fibrous attachment apparatus that inserts into the newly formed cementum.3
The Evolution of Regenerative Techniques
In 1957, Prichard provided "proof of principle" evidence that regeneration was not only theoretically possible but also clinically achievable in "ideal" periodontal defects with optimal conditions following subgingival debridement through a surgical approach.7 Since this early evidence of regeneration, research has focused on establishing clinical techniques to achieve optimal conditions in various clinical situations.3,7
The current techniques for periodontal regeneration that are being validated and researched include both mechanical (ie, guided tissue regeneration) and biologic approaches. The use of various bone grafting materials, such as demineralized or mineralized freeze-dried bone allograft (FDBA) and anorganic bovine bone graft (ie, xenograft), as well as growth factors and biologically active regenerative materials, such as enamel matrix derivative (EMD), recombinant human platelet-derived growth factor-BB (rhPDGF-BB), PepGen P-15, platelet-rich plasma, leukocyte- and platelet-rich fibrin (L-PRF), and recombinant human fibroblast growth factor-2 (rhFGF-2), have all been evaluated. Additional research is needed to further identify the benefits of these and other biologic agents for periodontal regeneration, including, but not limited to, the long-term effects, proper carriers, and ideal concentration or dosage, among other confounding factors.3,8
Two of the prominent factors for achieving periodontal regeneration are wound stability, which facilitates undisturbed blood clot adhesion and maturation on the instrumented root surface, and space provision, which enables the formation and maturation of periodontal tissues. In addition, ensuring that the healing period is uneventful serves to support the maturation of newly formed tissues.
Other influences that are driven by patient-based determinants and tooth-related considerations, such as diabetes mellitus, smoking, biofilm control, mobility, and defect morphology, can also negatively affect the success and outcomes of periodontal regeneration and have all been investigated. Future research is needed to further evaluate and emphasize patient-related predictors and determinants, individual response differences, patient-reported outcomes, and tooth-related conditions that may enhance treatment results.9
Consensus from the AAP
In 2014, the AAP held a workshop, Enhancing Periodontal Health Through Regenerative Approaches, that was charged with analyzing different methods of soft- and hard-tissue regeneration. The five areas evaluated were soft-tissue root coverage procedures, soft-tissue non-root coverage procedures, intrabony defects, furcation defects, and emerging regenerative approaches for periodontal reconstruction.
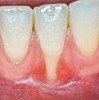
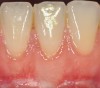
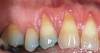
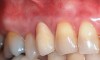
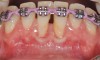
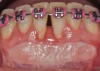
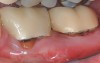
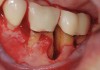
The workshop's group on periodontal soft-tissue root coverage procedures evaluated the predictability of root coverage procedures for single- and multiple-tooth Miller Class I and II10 periodontal recession defects. The workshop concluded that predictable root coverage was possible for Miller Class I and II recession involving a single tooth. When compared with the use of acellular dermal matrix graft (ADMG) (donor tissue) or EMD (porcine origin), procedures using a subepithelial connective tissue graft (SCTG) harvested from the patient's palate provided the best root coverage outcomes in conjunction with a coronally advanced flap.11 As alternatives to autogenous donor tissue, the workshop found strong evidence to support the use of an ADMG or EMD in conjunction with a coronally advanced flap and limited evidence to support the use of platelet-derived growth factor and xenogeneic collagen matrix.11 In addition, root coverage procedures were found to be effective for Miller Class I and II recession defects affecting multiple teeth, although the evidence is limited.11 Figure 1 and Figure 2 show the pretreatment and 1-year postoperative views of a soft-tissue root coverage treatment with SCTG and EMD that used a coronally advanced flap and a tunneling procedure. Figure 3 and Figure 4 depict the pretreatment and 3-year postoperative views of a root coverage procedure with ADMG and EMD that used a coronally advanced flap and a tunneling procedure (this patient was noncompliant following surgery and did not return to the office until the 3-year postoperative appointment).
Although similar to the root coverage procedures mentioned above, gingival augmentation procedures not aimed at achieving root coverage are performed to facilitate plaque control, improve patient comfort, and prevent future periodontal recession. They may be used in conjunction with restorative, orthodontic, or prosthetic dentistry. The consensus report from the AAP's workshop group on soft-tissue non-root coverage procedures concluded that a specific minimum amount of keratinized tissue is not needed to prevent attachment loss when optimal plaque control is present; however, if plaque control is suboptimal, a minimum of 2 mm of keratinized tissue is needed. 12 A standard procedure that is recognized to predictably gain keratinized tissue is the use of an autogenous gingival graft.12 Figure 5 through Figure 8 demonstrate the healing progression of a patient with poor oral hygiene whose mucogingival defect was treated with a free gingival graft and L-PRF from the patient's own blood.
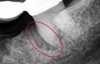
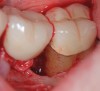
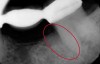
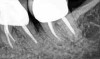
The consensus report from the AAP's workshop group on intrabony defects presents evidence that periodontal regeneration in intrabony defects is possible on previously diseased root surfaces, demonstrated by gains in clinical attachments, reductions in periodontal pocket depths, gains in radiographic bone heights, and overall improvements in periodontal health.9 These clinical findings are consistent with available histologic evidence, and the clinical improvements can be maintained over long periods of time (ie, > 10 years).9 Although bone replacement grafts have been a commonly investigated modality, guided tissue regeneration, biologics, and combination therapies have also been shown to be effective. Early management offers the greatest potential for successful periodontal regeneration.9 Figure 9 through Figure 11 depict the treatment of a tooth with probing depths of equal to or greater than 15 mm using mineralized FDBA.
Regarding furcation defects, based on the available evidence, the AAP workshop concluded that regenerative therapy is a viable option to achieve predictable outcomes for their treatment in certain clinical scenarios.13 Periodontal regeneration has been established as a viable therapeutic option for the treatment of various furcation defects, including Class II defects, which represent a highly predictable scenario. The application of combined therapeutic approaches (ie, barrier, bone replacement graft with or without biologics) appears to offer an advantage over monotherapeutic algorithms.13 Figure 12 through Figure 15 depict the periodontal regenerative treatment of two previously restored teeth with intrabony and furcation defects using mineralized FDBA and EMD.
What's Next?
To enhance treatment results, future research on regenerative approaches for periodontal reconstruction should emphasize patient-reported outcomes, individual response differences, and emerging technologies. The clinical selection and application of a particular regenerative therapy should be based on a clinician's experiences and understanding of the regenerative biology and technology. The decision-making process should take into consideration the potential adverse influence of any related factors, such as smoking, poor oral hygiene, tooth mobility, and defect morphology. For long-term success, management should be coupled with an effective maintenance program.9
Numerous emerging regenerative approaches for periodontal hard- and soft-tissue regeneration are being evaluated. Examining the cost-benefit ratio and assessing potential safety issues are important factors when considering the implementation of new, emerging therapies. Examples of emerging treatments include the application of protein and peptide therapy, cell-based therapy, genetic therapy, scaffolds, bone anabolics, and lasers. Other innovative approaches include therapies directed at the resolution of inflammation, treatments that take into account the influence of the microbiome, therapies involving the local regulation of phosphate and pyrophosphate metabolism, and treatments directed at harnessing the potential of existing therapies that are used for other purposes. As emerging technologies, most of these therapies lack high levels of evidence; therefore, additional research, evaluation, and analysis are necessary.14,15
In summary, periodontal hard- and soft-tissue regeneration techniques are viable, predictable treatment options for patients that can enhance the preservation of the architecture of the surrounding bone and supporting structures, ultimately preventing the loss of natural dentition. The provision of careful postoperative care and subsequent, supportive periodontal therapy is essential to achieve sustainable long-term regenerative outcomes. Notable elements to success include careful case selection and treatment planning, which should consider patient, tooth, site, and surgical factors in order to optimize patients' outcomes.3 To continue enhancing treatment results, it is imperative that future research be conducted to evaluate the efficacy of these novel regenerative approaches.4,14,15
About the Author
Jennifer Hirsch Doobrow, DMD
Diplomate
American Board of Periodontology
Fellow
International College of Dentists
Private Practice
Cullman, Alabama
References
1. Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-920.
2. Sculean A. Current clinical concepts in regenerative periodontal therapy. Perio Insight website. www.efp.org/perioinsight. Published Autumn 2017. Accessed January 9, 2019.
3. Ivanovski S, Bartold PM, Gronthos S, et al. Periodontal tissue engineering. In: Waddington RJ, Sloan AJ, eds. Tissue Engineering and Regeneration in Dentistry: Current Strategies, 1st ed. Hoboken, NJ: Wiley-Blackwell; 2017:124-144.
4. Caton JG. Overview of clinical trials on periodontal regeneration. Ann Periodontol. 1997;2(1):215-22.
5. The American Academy of Periodontology. Glossary of Periodontal Terms. The AAP Website. https://members.perio.org/libraries/glossary?_ga=2.118048521.1007904148.1547824583-993589543.1547824583&ssopc=1. Accessed January 9, 2019.
6. Reddy MS, JeffCoat MK. Methods of assessing periodontal regeneration. Periodontol 2000. 1999;19:87-103.
7. Prichard J. Regeneration of bone following periodontal therapy; report of cases. Oral Surg Oral Med Oral Pathol. 1957;10(3):247-252.
8. Suárez-López Del Amo F, Monje A, Padial-Molina M, et al. Biologic agents for periodontal regeneration and implant site development. Biomed Res Int. 2015;2015:957518. doi: 10.1155/2015/957518.
9. Reynolds MA, Kao RT, Camargo PM, et al. Periodontal regeneration - intrabony defects: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86(2 Suppl):S105-107.
10. Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):
8-13.
11. Tatakis DN, Chambrone L, Allen EP, et al. Periodontal soft tissue root coverage procedures: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86(2 Suppl):S52-55.
12. Scheyer ET, Sanz M, Dibart S, et al. Periodontal soft tissue non-root coverage procedures: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86(2 Suppl):S73-76.
13. Reddy MS, Aichelmann-Reidy ME, Avila-Ortiz G, et al. Periodontal regeneration -furcation defects: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86(2 Suppl):S131-133.
14. Cochran DL, Cobb CM, Bashutski JD, et al. Emerging regenerative approaches for periodontal reconstruction: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86(2 Suppl):S153-156.
15. Rios HF. Bashutski JD, McAllister BS, et al. Emerging regenerative approaches for periodontal reconstruction: practical applications from the AAP regeneration workshop. Clin Adv Periodontics. 2015;5(1):40-46.