You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The sinus cavity represents one of the least vascularized areas of the oral maxillofacial region, and as a result, augmentation procedures have generally been associated with lengthier healing times and higher failure rates.1,2 Not only is the rate of new bone formation within the area diminished, thereby increasing the length of standard sinus augmentation protocols, but a decreased amount of defense-fighting immune cells (white blood cells) in the area results in higher rates of infection.3,4
While sinus elevation procedures generally are routinely augmented with bone grafting materials, various platelet concentrates have increasingly been proposed as a means to speed revascularization to the sinus. Pioneered by Marx et al, platelet-rich plasma (PRP) was the first proposed therapy introduced for regenerative procedures in the field of oral maxillofacial surgery.5,6 PRP contains several growth factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-ß), among others, that are concentrated at 6 to 8 times normal physiological doses.7,8 While PRP has been used extensively in the field of oral maxillofacial surgery for regenerative procedures (also combined with bone marrow stromal cells), two main drawbacks have commonly been reported. First, the protocols to prepare PRP are lengthy, requiring two centrifugation cycles sometimes lasting up to 30 minutes. Second, to prevent coagulation during the lengthy centrifugation cycles, additional use of anticoagulants is necessary, altering the natural wound healing process.
For these reasons, Choukroun et al in 2001 proposed a second-generation platelet concentrate, platelet-rich fibrin (PRF), with complete anticoagulant removal.9 Because anticoagulants are not used, centrifugation must take place immediately for 8 to 12 minutes (ie, one centrifugation cycle). After centrifugation, red blood cells are excluded from the upper yellow plasma layer containing PRF with highly concentrated platelets, leukocytes, and growth factors.10 Growth factors released from PRF demonstrate a slow and gradual release over time when compared with PRP, which releases most growth factors within the first few minutes/hours. PRF's long-term release of growth factors has been shown to significantly improve cellular behavior, including cell migration and proliferation and differentiation of various cell types.11
Because of the biological advantages of PRF, it recently has often been used for sinus augmentation procedures both as a sole grafting material and in combination with bone grafts.12,13 While it is now basically confirmed that PRF can improve regeneration of the sinus cavity, the issue clinicians often face is whether to use PRF alone or in combination with a bone grafting material for sinus augmentation procedures.
Sinus Augmentation With Platelet-Rich Fibrin
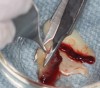
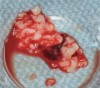
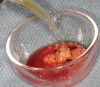
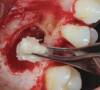
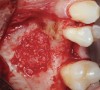
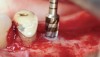
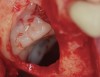
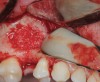
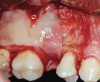
The biological advantages of using PRF for sinus augmentation procedures have been well-documented.14 PRF serves as a scaffold capable of protecting the Schneiderian membrane, may be combined with bone grafting materials to improve their handling and stability, and has been shown to increase new blood flow to the poorly vascularized area of the sinus cavity (Figure 1 through Figure 3). As a result, PRF is commonly used for all sinus augmentation procedures. The size of the sinus cavity has been the primary deciding factor for when to use PRF alone versus combined with a bone grafting material.
To date, various studies have demonstrated the use of PRF as a sole grafting material, with several reports demonstrating bone augmentations greater than 7.5 mm from the floor of the sinus to the apex of the implant.15-17 However, these reports have been criticized regarding their protocols, lack of appropriate controls, and limited available information regarding patient selection in comparison to other regenerative modalities. Furthermore, most studies to date have been presented as case reports and case series, rarely describing the importance or impact of implant loading protocols or, more importantly, the size of the sinus (most notably the bucco-palatal width of the sinus) prior to implant placement.
Therefore, when augmenting lost or missing bone in the sinus with PRF the clinician must take several essential factors into account. First, if PRF is to be used as a sole grafting material, implant placement mustbe performed simultaneously.18,19 If PRF is used as a sole grafting material for sinus augmentation utilizing a delayed approach without being combined with a bone grafting material or an implant surface, resorption will quickly follow with limited to no new bone formation occurring as a result of the relatively fast resorption rate of PRF of approximately 10 to 14 days.11 This is due to the limited osteoinductive properties of PRF, as its main function is to rapidly stimulate new blood flow, not new bone formation; therefore, absence of an osteoconductive implant surface or bone grafting material will result in little to no new bone formation. Additionally, the implant geometry acts as a space creator to provide needed volume for bone tissue ingrowth. These findings highlight the many studies and recent systematic review that have shown that within the sinus cavity, formation of a blood clot alone around an implant surface may result in new bone if primary stability is achieved.20-23
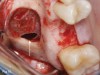
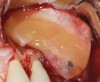
One reported limitation of using PRF alone for sinus augmentation procedures is its use in wide sinuses. Avila et al demonstrated that lateral sinus augmentation procedures performed with an allograft in narrow sinuses (<10 mm) and medium sinuses (10 mm to 15 mm) demonstrated roughly three times more vital bone after a 6-month healing period when compared with wide sinuses (>15 mm) (Figure 4).24 In response to these findings and the clinical experiences of numerous oral surgeons using PRF alone for sinus augmentation procedures, it has been recommended that sinuses >15 mm be regenerated in a combination approach with a bone grafting material. Therefore, all sinuses greater than 15 mm should be regenerated using PRF cut into small fragments with a bone grafting material (Figure 5 and Figure 6).
For narrow sinuses (<10 mm), PRF alone has been shown to lead to high success rates with predictable new bone formation taking place in the sinus routinely. Great interest remains, however, in investigating sinus augmentation procedures in medium sinuses, those between 10 mm and 15 mm. While reports and clinical case presentations have shown that PRF alone can be used in such cases, caution must be advised. Additional surgical modalities, such as new (osseodensification) surgical burs or sinus elevation kits, may be employed to optimize implant bed preparation and primary stability of implants (Figure 7 and Figure 8). Such strategies may be combined with PRF to enhance its use as a sole grafting material in slightly wider sinuses. Nevertheless, further research is necessary before such recommendations can be deemed predictable.
In 2015, a systematic review was conducted that evaluated the use of PRF for sinus elevation procedures.19 Of the 290 initial studies searched, the authors reported that only eight met the inclusion criteria with half not utilizing appropriate controls to compare their findings.19 The authors further noted that there was considerable heterogeneity in the results due to the major reported differences in the surgical techniques used (lateral sinus augmentation versus crestal approach), time of implant placement (simultaneous versus delayed), outcomes measured, biopsy analysis, and whether histological evaluation was performed.19 Based on this variability, it remains difficult to identify the "ideal" treatment protocol utilizing PRF, and, as a result, conservative clinical recommendations must be given.
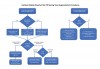
A decision-making flow chart is provided in Figure 9. If the sinus is <10 mm in width, PRF alone may be used to predictably regenerate it. When the sinus is >15 mm, PRF combined with a bone grafting material is advised. When the bucco-palatal dimension is between 10 mm and 15 mm, though it is advisable to combine PRF with a bone grafting material, the clinician's experience and the surgical protocols used may indicate the regenerative procedure. Nevertheless, future research is eminently needed to determine optimal conditions to further characterize when to use PRF alone versus in combination with a bone grafting material. Conservative, predictable protocols, as suggested in Figure 9, are always recommended.
The author's preferred choice of bone particulate graft is a combination of mineralized allograft (freeze-dried bone allograft [FDBA] - 50/50 mix of corticocancellous graft) mixed with a nonresorbable xenograft in a 1:1 ratio. FDBA induces new bone formation, while the nonresorbable xenograft holds volume.
Platelet-Rich Fibrin as Sole Material for Schneiderian Membrane Perforations
Another frequent use of PRF has been for the repair of Schneiderian membrane perforations (Figure 10 and Figure 11). While major advancements have been made with respect to surgical techniques, tools, and instruments to decrease the rate of membrane perforation, typical frequencies of tears generally have been reported in the 20% range.25,26 Traditionally, absorbable collagen membranes have been used most commonly; however, more recently attempts have been made to cover perforations with PRF. Due to the typically "sticky" consistency of PRF, this 100% natural fibrin scaffold may be used as a low-cost alternative to standard collagen membranes. Because of its natural properties, PRF, unlike a collagen barrier membrane, will not induce a foreign-body reaction.27
Figure 11 demonstrates a small Schneiderian membrane tear that was regenerated with PRF alone. However, it is recommended that tears larger than 5 mm be treated with a standard approach utilizing a collagen membrane. Nevertheless, the advantage of using PRF for perforations ≤5 mm in diameter is that the reparative process may be achieved with the aid of improved handling properties due to the sticky consistency of PRF without inducing a foreign-body reaction that may occur in response to collagen-derived biomaterial. As a rule, multiple layers of PRF may be utilized for larger tears, at virtually no additional cost. Because typical resorption periods are 10 to 14 days, it is advised to use PRF in a double layer to assure complete coverage (Figure 9). This also provides the added benefit of minimizing the risk of potential sinusitis that may be caused by low bone volume as a consequence of dramatic pneumatization after implant placement, owing to the cellular leukocyte content found in PRF.
Platelet-Rich Fibrin to Close Lateral Window During Sinus Augmentation Procedures
Because PRF also has been shown to be highly active on soft tissues, attempts have been made to use it as a sole barrier membrane to close lateral windows during sinus augmentation procedures (Figure 12 and Figure 13).28,29 Two studies to date from separate groups investigated the use of PRF in comparison with a collagen barrier membrane as a replacement material for lateral window closure. In both studies, PRF was shown to lead to similar results when compared with a collagen barrier membrane.28,29 These studies investigated implant stability and new bone formation around implants and/or implant success rates. Nevertheless, it remains difficult to evaluate whether PRF should be used as a complete barrier to soft-tissue invasion because of the estimated 10- to 14-day resorption period.
Therefore, lateral window closure is most frequently performed with a collagen barrier membrane. However, it is well-known that PRF is able to stimulate angiogenesis of tissues,30,31 and its use leads to faster soft-tissue regeneration. For these reasons, to further hasten soft-tissue healing and reduce patient morbidity PRF can be used over the top of collagen barrier membranes for improved tissue regeneration (Figure 14).
Predictably Regenerating the SinusWith Platelet-Rich Fibrin
Regeneration of a pneumatized sinus following tooth loss is a frequent surgical procedure requiring lengthy treatment healing times due to low vascularization to the sinus cavity when compared with other tissues in the oral cavity. As a result, the use of PRF has been increasingly utilized to speed new blood flow, thereby promoting faster bone formation within the sinus. The purpose of this article is not to discuss the surgical concepts for sinus augmentation procedures (eg, when to perform a sinus augmentation immediately versus utilizing a delayed approach, or when to utilize a lateral window versus crestal approach for sinus augmentation procedures). Discussion over these surgical topics and regenerative approaches is extensive. Instead, this article's intent is to discuss when, where, and why to utilize PRF in the aforementioned clinical scenarios either alone or in combination with a bone grafting material or barrier membrane.
When a delayed approach for implant placement is necessary, PRF must always be combined with a bone grafting material due to the fast resorption time of PRF scaffolds. Under no circumstance in such cases can PRF be used alone without the use of a bone grafting material or an implant. When implant placement is performed simultaneous with sinus augmentation, the factor most predictive to decide when to utilize PRF alone versus in combination with a bone grafting material is the bucco-palatal width of the sinus cavity. When sinuses are wider than 10 mm, it is generally recommended to combine PRF with a bone grafting material, whereas for narrower sinuses less than 10 mm, PRF may be used predictably as a sole grafting material (Figure 9). Similarly, when perforation of the sinus is encountered, PRF may be used alone when the sinus tear is 5 mm or less, whereas its combination with a collagen barrier membrane is more often recommended when the sinus is larger than 5 mm (Figure 9).
When sinus augmentation is performed using a crestal approach, the recommendation is always to place two PRF membranes through the implant osteotomy (prior to the introduction of bone grafting material or implant placement) to further protect the Schneiderian membrane (Figure 15). PRF scaffolds not only act as a biomaterial that improves vascularization of the sinus but may also be used as a preventative modality to limit potential tears/perforations/problems within the sinus.
An additional advantage of combining PRF to regenerate the sinus is its high incorporation of leukocytes, a cell type responsible for not only secreting growth factors but also for fighting incoming bacterial infections.32 Various clinical studies have investigated the ability of PRF to reduce/prevent infection. For instance, in a controlled study utilizing PRF for the extraction of bilateral mandibular third molars, it was found that the use of PRF alone resulted in a 9.5-fold decrease in infection rates and dry sockets.33 Furthermore, the use of PRF (in particular, the leukocytes within PRF scaffolds) along with analgesics taken post-surgery has been shown to decrease perceived pain among patients.34
In summary, there is great interest to further implement PRF into regenerative protocols of the sinus. The guidelines presented in this article are introduced as a conservative surgical modality to maximize the regenerative outcomes in a predictable manner.
Conclusion
The use of PRF for sinus augmentation procedures has garnered significant interest in recent years as a low-cost biological agent capable of improving vascularization to the sinus cavity. This article summarized the clinical indications for when to utilize PRF alone versus in combination with a bone grafting material or collagen barrier membrane for sinus augmentation procedures, Schneiderian membrane tears, and closure of the lateral window.
Acknowledgment
The clinical cases shown throughout the article were provided by Dr. Pikos.
About the Authors
Richard J. Miron, Dr. Med. Dent., DDS, PhD
Adjunct Faculty, Department of Periodontology, Nova Southeastern University, Fort Lauderdale, Florida; Course Instructor, Advanced PRF Education
Michael A. Pikos, DDS
Clinical Associate Professor, Departments of Periodontics and Prosthodontics, University of Florida College of Dentistry, Gainesville, Florida; Founder and CEO, Pikos Institute, Tampa, Florida; Private Practice, Oral and Maxillofacial Surgery, Palm Harbor, Florida
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Chambrone L, Preshaw PM, Ferreira JD, et al. Effects of tobacco smoking on the survival rate of dental implants placed in areas of maxillary sinus floor augmentation: A systematic review. Clin Oral Implants Res. 2014;2w5(4):408-416.
2. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dentawl implants. A systematic review. Ann Periodontol. 2013;8(1):328-343.
3. Ghasemi S, Fotouhi A, Moslemi N, et al. Intra- and postoperative complications of lateral maxillary sinus augmentation in smokers vs nonsmokers: A systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2017;32(4):759-767.
4. Kozuma A, Sasaki M, Seki K, et al. Preoperative chronic sinusitis as significant cause of postoperative infection and implant loss after sinus augmentation from a lateral approach. Oral Maxillofac Surg. 2017;21(2):193-200.
5. Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646.
7. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353-2360.
8. Leach JK, Kaigler D, Wang Z, et al. Coating of vegf-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249-3255.
9. Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42(55):e62.
10. Miron RJ, Fujioka-Kobayashi M, Bishara M, et al. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng Part B Rev. 2017;23(1):83-99.
11. Fujioka-Kobayashi M, Miron RJ, Hernandez M, et al. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88(1):112-121.
12. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part v: Histologic evaluations of prf effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
13. Simonpieri A, Choukroun J, Del Corso M, et al. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte-and platelet-rich fibrin as sole grafting material: A six-year experience. Implant Dent. 2011;20(1):2-12.
14. Castro AB, Meschi N, Temmerman A, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation, and implant therapy. A systematic review. J Clin Periodontol. 2017;44(2):225-234.
15. Mazor Z, Horowitz RA, Del Corso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J Periodontol. 2009;80(12):2056-2064.
16. Simonpieri A, Choukroun J, Del Corso M, et al. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: A six-year experience. Implant Dent. 2011;20(1):2-12.
17. Tajima N, Ohba S, Sawase T, Asahina I. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants. 2013;28(1):77-83.
18. Kanayama T, Horii K, Senga Y, Shibuya Y. Crestal approach to sinus floor elevation for atrophic maxilla using platelet-rich fibrin as the only grafting material: a 1-year prospective study. Implant Dent. 2016;25(1):32-38.
19. Ali S, Bakry SA, Abd-Elhakam H. Platelet-rich fibrin in maxillary sinus augmentation: A systematic review. J Oral Implantol. 2015;41(6):746-753.
20. Chen TW, Chang HS, Leung KW, et al. Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: A 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg. 2007;65(11):2324-2328.
21. Duan DH, Fu JH, Qi W, et al. Graft-free maxillary sinus floor elevation: A systematic review and meta-analysis. J Periodontol. 2017;88(6):550-564.
22. Lambert F, Léonard A, Drion P, et al. Influence of space-filling materials in subantral bone augmentation: blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin Oral Implant Res. 2001;22(5):538-545.
23. Thor A, Sennerby L, Hirsch JM, Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: An evaluation of 20 patients treated with 44 astra tech implants. J Oral Maxillofac Surg. 2007;65(7):64-72.
24. Avila G, Wang HL, Galindo-Moreno P, et al. The influence of the bucco-palatal distance on sinus augmentation outcomes. J Periodontol. 2010;81(7):1041-1050.
25. Hernández-Alfaro F, Torradeflot MM, Marti C. Prevalence and management of schneiderian membrane perforations during sinus-lift procedures. Clin Oral Implants Res. 2008;19(1):91-98.
26. Schwartz-Arad D, Herzberg R, Dolev E. The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J Periodontol. 2004;75(4):511-516.
27. Chia-Lai PJ, Orlowska A, Al-Maawi S, et al. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin Oral Investig. 2018;22(4):1851-1863.
28. Bosshardt DD, Bornstein MM, Carrel JP, et al. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: Histologic and histomorphometric results. Int J Periodontics Restorative Dent. 2014;34(2):259-267.
29. Gassling V, Purcz N, Braesen JH, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: A preliminary study. J Craniomaxillofac Surg. 2013;41(1):76-82.
30. Yoon JS, Lee SH, Yoon HJ. The influence of platelet-rich fibrin on angiogenesis in guided bone regeneration using xenogenic bone substitutes: a study of rabbit cranial defects. J Craniomaxillofac Surg. 2014;42(7):1071-1077.
31. Kubesch A, Barbeck M, Al-Maawi S, et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets. 2018;1-12. doi:10.1080/09537104.2018.1445835. [Epub ahead of print]
32. Everts PA, Overdevest EP, Jakimowicz JJ, et al. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg Endosc. 2007;21(11):2063-2068.
33. Hoaglin DR, Lines GK. Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. Int J Dent. 2013:875380.
34. Bilginaylar K, Uyanik LO. Evaluation of the effects of platelet-rich fibrin and piezosurgery on outcomes after removal of impacted mandibular third molars. Br J Oral Maxillofac Implants. 2016;54(6):629-633.