You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Soft-tissue pre-prosthetic surgery encompasses a number of procedures, such as epulis and denture hyperplasia removal, soft-tissue tuberosity reduction, frenectomy, vestibular deepening, and others. This article focuses on a carbon dioxide (CO2) laser vestibular extension procedure performed in a patient with Klinefelter syndrome, which is caused by a chromosomal abnormality.
Vestibuloplasty is defined as any of a series of surgical procedures designed to restore alveolar ridge height by lowering the muscles attached to the buccal, labial, and lingual aspects of the jaws.1 This procedure is indicated in cases of insufficient vestibular depth that may result from atrophy of the alveolar ridge and/or high attachment of muscle or movable mucosa.2,3 Most vestibuloplasty techniques involve vestibular deepening on the buccal-labial side of the mandible. In some patients with severe alveolar bone resorption or high genioglossus and mylohyoid musculature attachment, the floor-of-the-mouth procedure could increase the lingual vestibular depth. In this case, extra attention should be paid to the underlying structures to preserve the function of the tongue and oropharyngeal musculature.4 The main goal of vestibuloplasty is to enlarge the denture-bearing area and to ensure the comfort, function, and stability of the denture.
A sufficient amount of alveolar bone should be present. Sometimes the severity of the alveolar bone resorption makes deepening of the vestibule impossible. In such cases, a bone graft may be required. Among other factors precluding vestibular deepening are the condition, amount, and/or composition of the mucosa. When extensive resorbing of the mandible or maxilla occurs, the amount of stable surface bone may be insufficient to allow for an adequate denture to be made. Muscles in the mandibular such as the mylohyoid and genioglossus can interfere with the stability of the lower denture. In cases of poor mucosa condition, soft-tissue autogenous grafting or allografting may be necessary.2 Vestibuloplasty performed with grafting is associated with numerous postsurgical complications. Moreover, the need for a graft makes certain surgical modalities inapplicable for vestibular deepening (ie, grafts do not take on lasered epithelium, likely due to the insufficient exudate for nourishing the graft5).
Prior to the vestibular procedure, taking a thorough history and performing a physical examination (visual, palpation, and radiograph) are necessary. Factors the clinician should consider include the patient’s age, physical condition, family history, the amount and condition of the mucosa and underlying bone, and placement and tension of adjacent musculature.
Surgical Modalities
Traditionally, vestibular extension has been performed with a scalpel.3,4,6 Neckel7 conducted a study comparing scalpel and CO2 laser vestibuloplasty. The results of the study indicated that both techniques ensured good vestibular depth gain. However, the patients receiving treatment with the CO2 laser reported less pain and discomfort postoperatively than those receiving treatment with the scalpel.
In another study, Haytac and Ozcelik8 compared patient perceptions after frenectomies performed with the CO2 laser and scalpel techniques. Patients perceived the CO2 laser surgery more positively in terms of postoperative pain and function than traditional scalpel surgery. The article concluded that “the CO2 laser offers a safe, effective, acceptable, and impressive alternative” to the scalpel.8 In the present authors’ opinion, one of the disadvantages of a scalpel procedure compared to a CO2 laser is intraoperative hemorrhage that needs to be managed.
In the past, electrosurgery has also been used for vestibuloplasty,9 but no modern studies support the use of this modality for vestibular extension. This is possibly due to the small thickness of the soft tissue and close proximity to the bone. In a study comparing thermal damages of the CO2 laser with those created by electrosurgery in different types of soft tissue, Pogrel and colleagues5 concluded that the relatively narrow width of thermal tissue necrosis makes the CO2 laser excision superior for histologic examination of excised specimens than those created by electrosurgery.
CO2 Laser Soft-Tissue Oral Surgery
Not all lasers are equally efficient at both tissue vaporization (ie, ablation, cutting) and coagulation. The difference is illustrated in the absorption spectra for main soft-tissue chromophores10,11 in Figure 1. Some dental laser wavelengths (approximately 3000 nm, such as erbium lasers) are well absorbed by the water-rich soft tissue and are excellent for ablation but not as efficient at coagulation.11 Other dental laser wavelengths (approximately 1,000 nm, such as diode and Nd:YAG lasers) are efficient at coagulation but inefficient at ablation12 because they are poorly absorbed by the soft tissue.
The 10,600-nm CO2 laser wavelength is efficient at vaporizing and coagulating the soft tissue simultaneously (Figure 1), although it does not perform as well as an erbium laser at ablation or a diode/Nd:YAG laser at coagulation. Most importantly, the CO2 laser’s coagulation depth closely matches the blood capillary diameters,11 as discussed in Wilder-Smith et al13 and illustrated in Figure 2.
Laser pulsing is as important as wavelength; for cutting, short and powerful pulses are superior to long and weak ones. In the physics of pulsed laser surgery, thermal relaxation time is an important concept.11,12 Thermal relaxation time depends both on the tissue’s light absorption coefficient (Figure 1) and the tissue’s thermal diffusivity, as first described by Einstein.14 The irradiated tissue is vaporized with the highest efficiency if the thermal relaxation time is much longer than the pulse duration. The most efficient cooling of the tissue adjacent to the ablated zone is achieved when the period between laser pulses significantly exceeds the thermal relaxation time. Such laser pulsing specifications are referred to as a super pulse. A super pulse (Figure 3) minimizes collateral thermal damage, which makes it an essential feature of any state-of-the-art soft-tissue surgical CO2 laser.11 The optimal combination of the CO2 laser wavelength and these specific pulsing parameters ensures char-free, scar-free, and bloodless surgery with uncomplicated healing.
Laser Beam Spot Size
Just as the sharpness of the steel blade defines the quality and ease of the cut for traditional surgery, the size of the laser beam focal spot determines the quality of the cut in laser surgery. The smaller (or sharper) the focal spot of the beam, the narrower and deeper the incision. A dull blade cannot produce a quality incision; similarly, an oversized laser beam spot cannot produce a precise and narrow incision.
For cutting, a 10,600-nm CO2 laser is maintained 1 mm to 3 mm away from the tissue and is moved at a “hand speed” of a few millimeters per second (Figure 4). For a rapid switch from cutting to photocoagulation, the laser beam can be defocused. Defocusing can be achieved by simply moving the handpiece away from the tissue (by approximately 10 mm for tipless laser handpieces), and “painting” the “bleeder” for enhanced hemostasis (Figure 4).
Laser Power Density and Depth of Incision
For a laser, the power density of the focused laser beam is equivalent to the mechanical pressure that is applied to a traditional scalpel blade. In other words, greater laser fluence11 (ie, greater power density, slower hand speed) results in greater depth and rate of soft-tissue removal. When repetitive pulses are scanned across the soft tissue, the fluence is defined by the pulse frequency and the handspeed; in other words, the depth of incision depends on laser power settings, spot size, and the surgeon’s handspeed10,15 (Figure 5).
Advantages of Soft-Tissue Oral Surgery With a CO2 Laser Efficient Hemostasis
The CO2 laser’s excellent hemostasis and coagulation (due to the close match between coagulation depth and gingival blood-vessel diameters) allows practitioners to perform surgery even on extremely vascularized areas. The clinician benefits from improved visibility of the surgical field, which allows for highly precise and accurate tissue removal. Due to the efficient hemostasis, intraoral surgical wounds often do not require suturing or surgical dressing and can be left to heal by secondary intention.16
Minimal Postoperative Swelling
Minimal postoperative swelling and edema can be expected with the CO2 laser due to the intraoperative closure of lymphatic vessels on the margins of the laser incision. Lymphatic vessels regenerate approximately 8 to 10 days after capillary vessel proliferation.17
Reduced Postoperative Pain
Although pain is generally difficult to evaluate, patients have reported moderate levels of pain with CO2 laser surgery and often do not require analgesics.18 In the study by Niccoli-Filho et al,19 patients reported minimal discomfort only during the first 24 hours after the CO2 laser surgery. Based on patient pain perceptions during frenectomies performed with a CO2 laser, Haytac and Ozcelic8 concluded that laser treatment was less painful than when performing the procedure conventionally with a scalpel.
In Neckel’s study,7 vestibuloplasty was performed on 40 patients, divided into two groups—one receiving a conventional blade and the other a CO2 laser. Both showed a similar increase in the vestibular height, but patients in the CO2 laser group reported less pain and discomfort. Strauss and Fallon20 and Deppe and Horch21 compared the recovery process following CO2 laser surgery, cryosurgery, and electrosurgery and reported healing was faster and less painful with the CO2 laser.
Healing and Less Risk of Scarring
Minimized wound contraction and reduced risk for scar formation are among the biggest advantages of CO2 laser surgery.16,22-24 Healing of CO2 laser-irradiated wounds is characterized by a higher proliferation of fibroblasts that actively produce collagen. The findings of several studies24-26 indicate that in comparison with scalpel wounds, only a small number of myofibroblasts are present in the CO2 laser surgical sites; reduced count of myofibroblasts results in minimal wound contraction and scar formation or fibrosis in laser-treated areas.27 Seventy-two hours after CO2 laser surgery, the superficial necrotic layer of the laser-irradiated site is replaced by a fibroserous membrane.28,29 Approximately 2 weeks after surgery, wound epithelialization begins from the periphery toward the center. The epithelial covering of the laser wound is parakeratotic; it is also thinner than the epithelium that forms after scalpel resection. The aforementioned factors could explain the good cosmesis after CO2 laser surgery, which is characterized by smooth, elastic, new tissue and no fibrosis or scarring, whereas conventional surgery can result in some scarring.23 The combination of diminished wound contraction, minimized lateral tissue damage, precise control over the depth of incision, and excellent hemostatic efficiency makes the CO2 laser a safe and effective alternative to the use of a traditional scalpel.
Case Presentation
A 30-year-old patient with Klinefelter syndrome (KS) requested a complete denture prosthesis. One of the most common chromosomal disorders in humans, KS is characterized by hypogonadism and genetically determined infertility.30,31 KS affects only men, occurring in individuals who have two or more X chromosomes (eg, 47,XXY or 48,XXXY instead of 46,XY). KS is associated with cognitive disorders, osteoporosis, taurodontism, and dentofacial abnormalities.32 In cases of a rare variant of KS (49,XXXXY), permanent dentition may be completely absent.33
The patient was high functioning but had a cognitive developmental disorder. Visual examination revealed bilateral microtia and hemifacial microsomia associated with this genetic disorder. He displayed minimal anxiety.
Examination and Initial Findings
The examination consisted of visual intraoral and extraoral inspection and palpation followed by radiography. The following areas were assessed: the presence of soft- or hard-tissue pathologies, the relationships of the jaws and remaining teeth, the amount and condition of the alveolar bone of soft tissue covering the denture-bearing area, the contour of the alveolar ridge, vestibular depth, and muscle attachments.
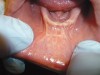
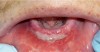
The examination revealed that the patient’s mandibular muscle attachment extended onto the crest of the ridge (Figure 6). The muscle fibers created considerable tension. Radiographic findings showed mild alveolar bone resorption as well. Such muscle placement and condition of the bone made the mandibular vestibule space inadequate for substantial denture foundation and inhibited fabrication of a complete denture. The patient’s bilateral lingual tori had been previously removed, which provided adequate vestibular height on the lingual side of the mandibular ridge. Therefore, lingual vestibular extension was not required. To take a good impression and ensure a comfortable fit with satisfactory retention and stability of the denture, it was decided to perform buccal-labial vestibular extension with the CO2 laser. The crest of the ridge and the soft-tissue condition did not require any grafting.
Procedure Equipment and Anesthesia
The procedure was performed in 1 session with a 10-W, 10,600-nm CO2 dental laser with an angled tipless handpiece. A 27-gauge needle and 5-0 chromic catgut sutures were used. The laser was set to a focal laser spot of 0.25-mm diameter and a power setting of 4 W. A super pulse laser exposure mode was used (repeat pulse F2-3, 29 Hz, 15 msec, 44% duty cycle), and the average power to tissue was 1.76 W (ie, 44 % of 4 W). Anesthesia consisted of 2 carpules of 4% articaine with 1:100,000 epinephrine and bilateral mental nerve blocks.
Technique
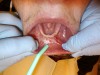
The clinician pulled the lower lip outward to maintain tension and facilitate soft-tissue incision. A horizontal CO2 laser incision was made starting at the left second premolar area and continued along the mucogingival junction line toward the second premolar of the contralateral side. The clinician made sure to extend the incision to the level of, but not through, the periosteum (Figure 7). Importantly, the trajectory of the incision was parallel to the bone surface. The small penetration depth of the CO2 laser wavelength enabled the clinician to remove very thin layers of tissue without collateral thermal changes or mechanical trauma to the adjacent lateral and underlying tissues. Severing thicker muscle fibers required multiple laser passes rather than a single incision.
The small penetration depth is important for the vestibular extension procedure, because it gives the surgeon precise control over the depth of the incision. The surgeon, therefore, is able to deepen the incision until the level of periosteum is reached without inadvertently cutting through it.
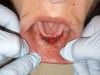
The freely movable mucosa flap was then carefully lowered to the level of the deepened vestibule and immobilized with resorbable sutures. The patient did not have a preexisting denture to hold retracted muscle tissue in the desired position. Therefore, the tissues were tacked down, as far apical as possible, using individual sutures. Connecting the suture in a continuous manner was not an option. If one had separated the tissue, it might have folded back. Sutures were left to resorb on their own (Figure 8). No bleeding was present, so no additional manipulations of the surgical site were needed.
Postoperative Care
The patient was released from the clinic immediately after the procedure and placed on amoxicillin (250 mg four times daily for 10 days). He was given vitamin E in gel form to place on tissue twice daily using an extremely gentle, nonabrasive 12,000-bristle surgical brush. The patient was also given an organic, alcohol-free irrigation product to debride the area before rinsing it with salt water twice daily. Dietary instructions included the restriction of rough or abrasive foods, such as potato chips or crackers.
Follow-up Examinations
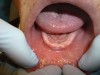
Follow-up examination 2 weeks postoperatively showed good epithelialization (Figure 9). Four weeks after the vestibular extension procedure, the surgical site had healed completely with smooth pliable tissue and no scarring (Figure 10). Impressions were taken 6 weeks after the surgery impressions.
The vestibule depth increased by 8 mm. The achieved vestibule height allowed for efficient stable denture placement (final delivery is shown in Figure 11). The patient was satisfied with the result.
Conclusion
The 10,600-nm CO2 laser offers numerous advantages over a conventional scalpel and other laser wavelengths for soft-tissue pre-prosthetic surgery, including the vestibular extension procedure. The benefits, which include speed, excellent hemostasis, absence of inflammation, reduced pain, and good patient acceptance, are especially important in patients with various developmental disorders, such as in the case reported in this article.
Acknowledgment
The authors greatly appreciate the support of and contribution from Anna Glazkova, PhD, in preparing this material for publication.
About the Authors
Robert Levine, DDS
Director of Laser Dentistry
Arizona School of Dentistry & Oral Health
Mesa, Arizona
Founder
Global Laser Oral Health LLC
Scottsdale, Arizona
Peter Vitruk, PhD, MInstP, CPhys, DABLS
Founder
LightScalpel LLC
Woodinville, Washington
Member
Science and Research Committee
Academy of Laser Dentistry
Coral Springs, Florida
Faculty
California Implant Institute
San Diego, California
Faculty
Global Laser Oral Health LLC
Scottsdale, Arizona
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Vestibuloplasty. Mosby’s Dental Dictionary. 2nd ed. 2008. http://medical-dictionary.thefreedictionary.com/vestibuloplasty. Accessed December 7, 2015.
2. Namour S. Atlas of Current Oral Laser Surgery. Boca Raton, FL: Universal Publishers; 2011:106-110.
3. Malik NA. Preprosthetic surgery. In: Malik NA, ed. Textbook of Oral and Maxillofacial Surgery. 3rd ed. New Dehli, India: Jaypee Brothers;2012: 467-490.
4. Convissar RA, Sawisch TJ, Strauss RA. Laser-enhanced removable prosthetic reconstruction. In: Convissar RA. Principles and Practices of Laser Dentistry. St. Louis, MO: Mosby; 2011:157-165.
5. Pogrel MA, McCracken KJ, Daniels TE. Histologic evaluation of the width of soft tissue necrosis adjacent to carbon dioxide laser incisions. Oral Surg Oral Med Oral Pathol. 1990;70(5):564-568.
6. Hashemi HM, Pharhiz A, Ghafari S. Vestibuloplasty: allograft versus mucosal graft. Int J Oral Maxillofac Surg. 2012;41(4):527-530.
7. Neckel CP. Vestibuloplasty: a retrospective study on conventional and laser operation techniques. Proc. SPIE 3593, Lasers in Dentistry V. 1999;76. doi:10.1117/12.348330.
8. Haytac MC, Ozcelik O. Evaluation of patient perceptions after frenectomy operations: a comparison of carbon dioxide laser and scalpel techniques. J Periodontol. 2006;77(11):1815-1819.
9. Forman D, Lieblich SE, Berger J, Gold BD. Use of the electrosurgical knife and topical thrombin for hemostasis in split-thickness skin graft vestibuloplasty. J Oral Maxillofac Surg. 1984;42(11):751-752.
10. Cobb C, Vitruk P. Effectiveness of a super pulsed CO2 laser for removal of biofilm from three different types of implant surfaces: an in vitro study. Implant Practice US. 2015;8(3):14-20.
11. Vitruk P. Oral soft tissue laser ablative and coagulative efficiencies spectra. Implant Practice US. 2014;7(6):22-27.
12. Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103(2):577-644.
13. Wilder-Smith P, Arrastia AM, Liaw LH, Berns M. Incision properties and thermal effects of three CO2 lasers in soft tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):685-691.
14. Einstein A. Über die von der molekularkinetischen Theorie der wärmegeforderten Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann Phys. 1905;322(8):549-560.
15. Linden E, Vitruk P. SuperPulse 10.6 um CO2 laser-assisted, closed flap treatment of peri-implantitis. Implant Practice US. 2015;8(4):31-34.
16. Zaffe D, Vitale MC, Martignone A, et al. Morphological, histochemical, and immunocytochemical study of CO2 and Er:YAG laser effect on oral soft tissues. Photomed Laser Surg. 2004;22(3):185-189.
17. Lambrecht JT, Stübinger S, Hodel Y. Treatment of intraoral hemangiomas with the CO2 laser [in French, German]. Schweiz Monatsschr Zahnmed. 2004;114(4):348-359.
18. Pogrel MA. The carbon dioxide laser in soft tissue preprosthetic surgery. J Prosthet Dent. 1989;61(2):203-208.
19. Niccoli-Filho W, Neves AC, Penna LA, et al. Removal of epulis fissuratum associated to vestibuloplasty with carbon dioxide laser. Lasers Med Sci. 1999;14(3):203-206.
20. Strauss RA, Fallon SD. Lasers in contemporary oral and maxillofacial surgery. Dent Clin North Am. 2004;48(4):861-888.
21. Deppe H, Horch HH. Current status of laser applications in oral and cranio-maxillofacial surgery. Med Laser Appl. 2007;22(1):39-42.
22. Zeinoun T, Nammour S, Dourov N, et al. Myofibroblasts in healing laser excision wounds. Lasers Surg Med. 2001;28(1):74-79.
23. Wang X, Ishizaki NT, Matsumoto K. Healing process of skin after CO2 laser ablation at low radiance: a comparison of continuous-wave and pulsed mode. Photomed Laser Surg. 2005;23(1):20-26.
24. Grbavac RA, Veeck EB, Bernard JP, et al. Effects of laser therapy in CO2 laser wounds in rats. Photomed Laser Surg. 2006;24(3):389-396.
25. de Freitas AC, Pinheiro AL, de Oliveira MG, Ramalho LM. Assessment of the behavior of myofibroblasts on scalpel and CO2 laser wounds: an immunohistochemical study in rats. J Clin Laser Med Surg. 2002;20(4):221-225.
26. Fisher SE, Frame JW, Browne RM, Tranter RM. A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch Oral Biol. 1983;28(4):287-291.
27. Desmoulière A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83(12):1689-1707.
28. Basu MK, Frame JW, Rhys Evans PH. Wound healing following partial glossectomy using the CO2 laser, diathermy and scalpel: a histological study in rats. J Laryngol Otol. 1988;102(4):322-327.
29. Tambuwala A, Sangle A, Khan A, Sayed A. Excision of oral leukoplakia by CO2 lasers versus traditional scalpel: A comparative study. J Maxillofac Oral Surg. 2014;13(3):320-327.
30. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364(9430):273-283.
31. Nieschiag E. Klinefelter syndrome: the commonest form of hypogonadism, but often overlooked or untreated. Dtsch Arztebl Int. 2013;110(20):347-353.
32. Joseph M. Endodontic treatment in three taurodontic teeth associated with 48,XXXY Klinefelter syndrome: a review and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):670-677.
33. Achtari MD, Achtaris M, Georgakopoulou EA. Dental treatment of 49,XXXXY syndrome: a case report. Hospital Chronicles. 2014;9(4):266-268.