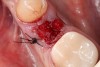
You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
One of the most common procedures performed daily in dental practices is the removal of hopeless or severely damaged teeth due to the presence of periodontal disease or non-restorable tooth decay. There is no doubt that many dentists who have been practicing for any significant amount of time have successfully removed thousands of teeth without any significant consequences. However, gauging the prognosis for the extraction site is important, no matter the plan.
In dentistry, the healing of the socket after the extraction of the tooth has become an important topic of research, study, and discussion.1 In addition, increasing patient demands for esthetics emphasizes how important it is to maintain adequate ridge volume to achieve a long-term esthetically acceptable prosthetic outcome, whether through implant-supported prosthetics or conventional restoration.2 Tooth extraction is initially perceived merely as tooth loss, but local changes lead to hard- and soft-tissue alterations.
The loss of bone after the extraction is a significant problem in both implant and restorative dentistry. Clinical studies have shown as much as a 32% change in the width of the alveolar ridge at 3 months and as much as a 69% change in width between 6 and 7 months. The ridge exhibits a limited reduction in its vertical dimension after single-tooth extraction, but the horizontal reduction is substantial. In terms of measurable bone loss, that translates to as much as 1 mm to 3 mm in alveolar ridge height and 3 mm to 5 mm in ridge width that may be lost due to the resorptive nature of the healing process.3 Non-grafted sockets generally resorb lingually, and the remaining buccal wall “melts” away, often leaving knife-edge ridges or large buccal wall defects that are difficult to restore esthetically.
The literature has shown that advanced socket management techniques combined with atraumatic tooth extraction can significantly reduce early bone loss. Bone grafts and substitutes were placed in the extraction socket to maintain the dimension of the ridge after tooth removal. Studies show that fresh extraction sockets in the maxillary anterior region that were grafted with a deproteinized bovine bone mineral demonstrated less loss of the buccal plate of the ridge than non-grafted control sites.4-6
Bone Formulation and Turnover
Bone morphology and bone healing have a cellular component. The four major cell types in the context of socket grafting are osteoprogenitor cells, osteoclasts, osteoblasts, and osteocytes.
1. Osteoprogenitor cells are undifferentiated stem cells that line the endosteal surfaces of bone. These cells will differentiate into pre-osteoblastic and mature osteoblastic cells lining the endosteal surface of bone.7
2. Osteoclasts are responsible for the resorptive aspect of bone modeling and remodeling.7
3. Osteoblasts are responsible for the deposition and calcification of the new bone matrix.7
4. Osteocytes are osteoblasts that have done their job and become embedded in the new bony matrix and play a role in cell-to-cell communication when the bone responds to load or injury.8
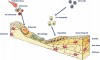
Two concepts used to describe this dynamic nature of bone are referred to as modeling and remodeling (Figure 1).9
Modeling is the process in which bone changes because of the response to stimulus or physical force.10 Remodeling refers to the constant internal turnover of bone in which the osteoblastic and osteoclastic activity is more harmonious and balanced so that the human skeleton is maintained in health.9
Types of Graft Material
Most often, the determining factor in socket-preservation bone-grafting procedures is the dentist’s ability to remove the hopeless tooth with as little damage to the surrounding hard and soft tissues as possible. The remaining bony walls of the socket will determine the type of graft material necessary to repair the defect. The mechanisms by which the graft materials heal determine which materials are most appropriate for each situation. Three characteristics to consider are whether the graft material is:
1. Osteoconductive: Analogous to a scaffold or framework on which existing bone cells may form, resulting in bone growth within a defect that may otherwise repair with soft tissue.
2. Osteoinductive: Materials that stimulate bone growth even in areas where bone would not typically form. An example would be the formation of bone in muscle tissue.
3. Osteogenic: The formation of new bone from living cells transplanted within the graft.
Several different types of graft material can be used when grafting an extraction socket. They are autografts, allografts, xenografts, and alloplasts.11 Autografts, or autogenous grafts, are transplanted within the same patient. Allografts are transplanted from a different patient but within the same species. Xenografts are derived from a different species. Alloplasts are synthetic and biocompatible.
The ideal bone graft material would be described as being bioresorbable, osteoconductive, osteoinductive, and osteogenic. An autograft is the only graft material that exhibits all of these properties.11 Autogenous bone by its very nature may not be the best option for the patient or may be available in limited quantities, making it necessary to have other readily available options for the treating dentist.
The most common graft materials used for socket preservation procedures are allografts (Table 1). They are generally easy to use, cost-effective, and readily available in three forms with several configurations and indications for use.
Configurations and Indications
Demineralized freeze-dried bone allograft (DFDBA) originates from human cadavers and is believed to be osteoinductive because of the presence of bone morphogenic proteins.7 Some advantages to using DFDBA are the predictability of bone formation, reasonable cost, and availability. The turnover time of DFDBA is relatively rapid compared with other allograft materials. Usually between 6 and 12 months is required for its resorption and replacement with vital bone.
Mineralized freeze-dried bone allograft (MFDBA) is mostly used in socket-grafting procedures prior to the placement of dental implants. Both osteoinductive and osteoconductive,2 MFDBA is available in several forms such as cortical, cancellous, and in blocks. Its disadvantage is the prolonged turnover time, which can be 6 months or more, longer than that of DFDBA. Generally, MFDBA can be used in larger defects due to its high strength, density properties, slower turnover time, and ability to maintain space during the healing phase.7
Often, a combination of DFDBA and MFDBA may also be used, enabling the clinician to take advantage of the properties of both materials such as the osteoconductivity and rapid turnover time of the demineralized material and the higher density and longer turnover time of the mineralized material. Used together, the two materials and their healing properties make it a good option for grafting procedures when implants are the treatment of choice for the replacement of the extracted tooth and when a rapid turnaround time is indicated or desired.12
Alloplasts are synthetic implantable biomaterials and are generally indicated for use when more conventional restorative options are desired and implants are not planned for the site.7 They can be mixed with autogenous graft materials to form a “combination” graft, which may result in better bone density and stability for a more complete fill of larger bone defects. They are osteoconductive and generally take between 6 and 24 months for resorption, depending on the material used and the conditions present in the grafted site. Many forms of alloplast are available, each with different properties and indications.
Xenografts are derived from another species. The most common forms are bovine bone and porcine collagen. Xenografts are osteoconductive and their structure, chemistry, and architecture are similar to human bone.
Types of Barrier Membranes
In normal healing of the extraction site, soft-tissue cells migrate at a much faster rate than bone cells. Therefore, larger defects are inclined to fill with soft tissue.13 It becomes critical to consider the use of a biocompatible barrier membrane in conjunction with the graft material used in the extraction socket. The primary role of any barrier membrane is the prevention of soft-tissue cell migration into the graft material during healing. Studies have shown that the use of a barrier membrane after extraction reduces the amount of crestal bone loss and less horizontal ridge resorption.14 Other key characteristics include biocompatibility, suitable occlusive properties, bio-inertness, ease of use, and resistance to infection if exposed to the oral cavity.
In general terms, two types of barrier membranes are available for socket-grafting procedures: resorbable and nonresorbable. However, several configurations of the two types exist, which will determine how and when they are indicated.
The most commonly used resorbable membranes are formed from a collagen-based matrix usually derived from bovine or porcine collagen, human dermis, or pericardium. The major determining factors in the selection of the type of membrane are the density of the collagen matrix and its ability to withstand exposure to the oral cavity environment. Resorption times can be unpredictable and will vary from patient to patient. Variations in resorption time can be attributed to the location of the surgical site, the ability to achieve primary closure, exposure to the oral cavity, and the density and type of collagen-based membrane selected.15
Another form of a collagen-based barrier is a collagen sponge or plug. These barriers resorb much faster than true collagen membranes and should only be considered when using smaller socket grafts with little complications. They are inexpensive and can help to reduce the costs of materials without jeopardizing the overall success of the graft.
Nonresorbable membranes are generally made of polytetrafluoroethylene (PTFE), also known as Teflon®, and are either high-density PTFE (d-PTFE) or expanded PTFE (e-PTFE).13 Their use is recommended when repairing larger defects or when primary closure cannot predictably be achieved and is indicated to protect the underlying graft material. The differences in these two types of membranes are simple. High-density PTFE can be used as a barrier membrane for larger defects when primary closure is difficult or impossible to achieve. The manufacturing characteristics of d-PTFE material allow it to remain exposed to the oral cavity while protecting the defect by decreasing the risk for bacterial contamination of the graft and ingrowth of soft tissue into the newly placed bone graft material. d-PTFE is soft, flexible, easy to handle, and can be easily removed without the use of overly invasive secondary surgical procedures.16
In contrast, the manufacturing characteristics of e-PTFE make it highly porous, which consequently results in a higher risk for bacterial infection when the membrane is exposed to the oral cavity during healing, as well as a higher chance for the ingrowth of soft tissue. This makes its removal a much more difficult secondary procedure. The use of e-PTFE membranes in socket preservation grafting is not indicated because of these characteristics.
Socket Defects
To successfully repair the extraction socket, the clinician must be able to determine the type of defect present.
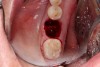
The ideal scenario for a socket preservation graft would be a single-wall defect, resulting from the atraumatic extraction of the tooth without any significant damage to the surrounding hard and soft tissues. In a single-wall defect, the only wall missing is the tooth itself, or the “roof” of the socket, resulting in an intact “bowl,” which greatly increases the success of the socket preservation procedure. This is the most common defect when performing the extraction of a single tooth (Figure 2).
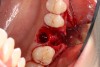
A two-wall defect is also common, usually resulting from the fracture of the buccal plate during the extraction of the tooth. As a result, a barrier membrane is necessary to contain the graft material and decrease the risk for soft-tissue invasion into the graft material (Figure 3).
Special care must be taken when selecting the graft material for three- and four-wall defects because of the general size and involvement. Most often, a “combination” graft, or a mixture of DFDBA and MFDBA or cortical/cancellous mixture of differing particle size, might be indicated in these cases because of the properties mentioned above in reference to grafting larger defects and the use of barrier membranes.17
Five-wall defects are rare, and referral to a specialist should be considered when presented with this type of defect.
Things to Consider When Choosing a Socket Graft Material
It is almost impossible to select one graft material suitable for all clinical situations. Based on the desired outcome of the final treatment plan, selection becomes part of the planning. The prosthetic options following the extraction of the tooth fall under three main categories: traditional fixed prosthodontics, removable prosthodontics, or implant-supported fixed or removable prosthetics. For each of these options, the graft material of choice may differ greatly based on the specific resorption characteristics of the material and its ability to support the final treatment plan.
When considering traditional fixed prosthodontics in which the extracted tooth becomes a pontic site, a dense, non-resorbable synthetic alloplast may be an appropriate material choice based on the desire for long-term stability of the alveolar ridge, ease of use, relatively low cost, and long-term clinical success, assuming that there is no desire to place an implant in that site in the future.
However, if an implant is expected to be placed and the socket is intact, the material of choice might be one that is rapidly resorbed and replaced with vital bone such as mineralized cancellous allograft. If the defect is larger as a result of the extraction, a history of trauma or infection is present, or if it is undecided whether an implant will be placed in the extraction site, a combination graft, as mentioned before, combined with a barrier membrane may be indicated. This type of graft would provide a more rapidly resorbed material with a stronger, more densely packed substance to help maintain the space during the healing phase, until the implant could be placed at a later time.12
Procedure and Techniques
Using proper techniques will ultimately allow the clinician to accomplish treatment goals and conclude a successful procedure:
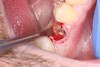
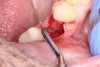
1. Minimally invasive, atraumatic extraction of the remaining tooth structure with the use of periotomes or similar instruments as well as sectioning of the tooth, when indicated, should be performed to minimize any unnecessary trauma to the thin cortical bone and surrounding soft tissues (Figure 4).
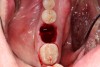
2. The remaining socket should be cleaned and curetted of all remaining granulation tissue or residual soft tissue present at the apex, especially with endodontically treated teeth (Figure 5).
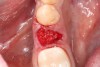
3. Make sure the socket is bleeding (Figure 6). If necessary, decorticate the remaining socket walls, using a round bur or surgical-type bur, to create a bleeding wound that will facilitate early vascularization and the beginning of the primary healing cascade.
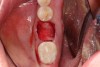
4. Ensure that the selected graft material is properly hydrated, usually with normal sterile saline, for 15 to 20 minutes prior to delivering to the fresh, bleeding extraction site (Figure 7).
5. Whether choosing a PTFE or a collagen membrane, start by creating a subperiosteal pocket with a small elevator or curette on the facial and lingual aspects of the remaining socket that extends 3 mm to 5 mm past the margins of the socket or remaining defect.
6. Deliver the graft material to the socket, and gently condense the material into the socket. Ensure that the material is evenly distributed into the socket and that no large voids in the graft are present. Do not condense the material or pack it too tightly into the defect. This reduces the space for vascularization and may increase the patient’s postoperative discomfort as well as delay healing of the graft material (Figure 8).
7. Tuck the membrane or collagen sponge into the facial or lingual aspect of the pocket, creating an “envelope” that will be closed over the graft material. Take care to ensure the membrane lies flat against the alveolar bone and is not crimped or folded after placement (Figure 9).
8. Stabilize the membrane or collagen sponge with a criss-cross mattress suture, taking care not to suture through the membrane. Subsequent interrupted sutures may be used to achieve adequate closure, but take care not to oversuture the flap, which can result in sloughing of the tissue and loss of the membrane and graft material (Figure 10).
9. Remove the suture in 10 to 14 days.
10. When a d-PTFE membrane is used for coverage of the graft material, it can be removed at 21 to 28 days. Studies have shown that at this point, a dense, vascular connective tissue matrix in the socket is present and that early osteogenesis has begun in the apical two-thirds of the socket.18
11. The grafted socket and soft tissue is allowed to heal and mature for a minimum of 6 weeks when the final treatment plan involves traditional fixed prosthodontics and a minimum of 4 months when considering implant placement (Figure 11).
Though there are always advances in this field, platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are the most recent developments for use in grafting procedures, to the author’s knowledge. In simple terms, the use of PRP and PRF require the ability to draw the patient’s blood, spin the platelets and/or the fibrin out in a centrifuge, and manipulate the separated particles into a pseudo-membrane or plug that is mixed with the graft particulate and/or to seal the graft sight. The author believes that these concepts are beyond the scope of this article in terms of performing socket preservation in the typical general practitioner’s practice.
Conclusion
Socket preservation grafting can play a vital role in the successful replacement of a missing tooth whether using conventional fixed or implant-supported prosthodontics. Learning the techniques of atraumatic extraction in order to preserve the hard and soft tissues and the rationale behind the selection of the correct material for the socket graft dramatically increases the chance for success of the grafting procedure. The results are more esthetic, longer-lasting, hygienic outcomes. Preserving the integrity of the alveolar process is critical to the overall success of treatment plans. The procedure, when managed correctly, is relatively easy to learn and to implement in general dental practice.
About the Author
Scott Thomas Keys, DDS, has been practicing dentistry since 1996 and has completed over 3,000 hours of postgraduate continuing education in the areas of bone grafting and ridge preservation, implant surgery and restoration, cosmetic dentistry and full-mouth reconstruction, and occlusion.
Disclosures
Scott Thomas Keys, DDS, has no relevant financial relationships to disclose.
References
1. Araújo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontology 2000. 2015;68(1):122-134.
2. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25(2):113-119.
3. Lang NP, Pun L, Lau KY, et al. A systemic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin Oral Implants Res. 2012;23(Suppl 5):39-66.
4. Vignoletti F, Matesanz P, Rodrigo D, et al. Surgical protocols for ridge preservation after tooth extraction. A systemic review. Clin Oral Implants Res. 2012;23(Suppl 5):22-38.
5. Nevins M, Camelo M, De Paoli S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19-29.
6. Sahlin-Platt A. Bone tissue regeneration in dentoalveolar surgery. Clinical and experimental studies on biomaterials and bone graft substitutes. Odontological Dissertations, Series No. 119. Department of Odontology, PhD thesis, Umea University, Sweden.
7. Bartee BK. Extraction site reconstruction for alveolar ridge preservation. Part 1: rationale and materials selection. J Oral Implantol. 2001;27(4):187-193.
8. Ten Cate AR. Oral Histology: Development, Structure, and Function. 3rd ed. St. Louis, MO: Mosby;1989:117.
9. Trombelli L, Farina R, Marzola A, et al. Modeling and remodeling of human extraction sockets. J Clin Periodontol. 2008;35(7):630-639.
10. Devlin H, Ferguson MW. Alveolar ridge resorption and mandibular atrophy. A review of the role of local and systemic factors. Br Dent J. 1991;170(3):101-104.
11. Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993;2(3):158-167.
12. Becker W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sites. J Periodontol. 1994;65(12):1128-1133.
13. Bartee BK. Extraction site reconstruction for alveolar ridge preservation. Part 2: membrane-assisted surgical technique. J Oral Implantol. 2001;27(4):194-197.
14. Tischler M, Misch CE. Extraction site bone grafting in general dentistry. Review of applications and principles. Dent Today. 2004;23(5):108-113.
15. Gottlow J. Guided tissue regeneration using resorbable and non-resorbable devices: initial healing and long-term results. J Periodontol. 1994;64(11 Suppl):1157-1165.
16. Kirkland G, Greenwell H, Drisko C, et al. Hard tissue ridge augmentation using a resorbable membrane and a particulate graft without complete flap closure. Int J Periodontics Restorative Dent. 2000;20(4):382-389.
17. Misch CE. Bone augmentation for implant placement: keys to bone grafting. In: Contemporary Implant Dentistry. 2nd ed. St. Louis, MO: Mosby;1999:451-468.
18. Greenstein G, Carpentiere, J. Utilization of d-PTFE Barriers for Post Extraction Bone Regeneration in Preparation for Dental Implants. Compend Contin Educ Dent. 2015;36(7):465-473.