You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Mary A. Borg-Bartlett Reducing exposure to bloodborne pathogens not only creates a safer and more healthful workplace, but also reduces costs while increasing productivity and employee morale. The Occupational Safety and Health Administration (OSHA) requires that employers protect workers who could be exposed to bloodborne pathogens, as well as eliminate or minimize these exposures. Becoming more familiar with the Bloodborne Pathogens Standard and the guidelines established by the Centers for Disease Control and Prevention (CDC) will help employers develop an effective Exposure Control Plan. The purpose of this article is to review the requirements set out by OSHA’s Bloodborne Pathogens Standard and discuss how those requirements pertain to possible exposures in dental laboratories.
As stated in an OSHA Fact Sheet, “Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), the virus that causes AIDS. Workers exposed to bloodborne pathogens are at risk for serious or life-threatening illnesses.”1 The Bloodborne Pathogens Standard can be found in its entirety on OSHA’s website (www.osha.gov) as regulation 29 CFR 1910.1030.2 This 16-page document was adopted by OSHA on December 6, 1991 and became effective in 1992. OSHA requires employers who have workers with these risks to comply with the Bloodborne Pathogens Standard. Let’s review the requirements that this document sets out.
Establish an Exposure Control Plan
OSHA provides employers with a model Exposure Control Plan via their website,3 and the following details a written plan that establishes how an employer can eliminate or minimize potential occupational exposure to bloodborne pathogens. In order to prepare the exposure determination, the employer must:
Assess the workplace for types of exposure.
Prepare a list of job classifications in which all of the workers have occupational exposure (referred to as Category I workers) and a list of job classifications in which some of the workers have occupational exposure (referred to as Category II workers).
Prepare a list of the tasks and procedures performed by each of those workers.
The assessment stated in Item 1 is to be performed by employers to determine the areas in which it can be reasonably anticipated that workers will come into contact with blood or other potentially infectious materials (OPIM) while performing regular job duties.
The list of job classifications stated in Item 2 can be a list by job title. However, this can be difficult in a dental laboratory because not all positions have titles that are descriptive of the risk areas. Therefore, it makes more sense for a dental laboratory to prepare the list in the form of job tasks, as stated in Item 3. Safety procedures must also be prepared to identify the procedures workers must observe to protect them from OPIM.
The following details areas that may pose an exposure risk in dental laboratories.
Pickup and Delivery Personnel
Pickup and delivery personnel who enter the dental offices must be trained on how to handle deliverables in a way that prevents exposure during transport. If a dental team member tries to hand a pickup and delivery person an impression—or any other item that has been in a patient’s mouth—that person should refuse to handle the item and ask the dental team member to package it for safe transport.
Receiving Personnel
Receiving personnel in the dental laboratory will typically unpackage deliverables, prepare appropriate paperwork, perform disinfection procedures, and send the new items to either the scheduling or production departments. These individuals must be trained on how to safely handle items during the disinfection process, and they should always wear a mask, gloves, gown, and safety eyewear or face shield while performing disinfecting procedures. Please note that it is not uncommon for items received from the dentist to contain blood, so it is paramount that receiving personnel know how to remove blood prior to disinfection. Blood can be particularly visible on implant components, such as the items pictured in Figure 1. The laboratory should communicate with the clinical practice to make them aware of any concerns and ask for their assistance in removing blood and other bioburden prior to shipping items to the dental laboratory.
Model Room Workers
When clinically poured models are received from dental clients, steps need to be taken to disinfect all equipment and areas contaminated when die trimming or using the model trimmer. Additionally, model room workers must be aware of other potentially infectious materials (OPIM) when grinding on clinically poured models. Whenever possible, employers should install engineering controls to eliminate the need for personal protective equipment. One solution for protecting workers grinding on clinically poured models would be to place the model trimmer in a shatter-proof box equipped with suction. Most debris from grinding will be captured inside the box; however, it is still advisable for the worker to wear safety eyewear as a precaution. Figure 2 shows a worker at Specialty Appliances Dental Laboratory in Cumming, GA using engineering controls for protection during model grinding.
Another infection risk in the model department occurs when technicians trim back over-extended borders on impressions. These personnel must wear personal protective equipment when performing these tasks, such as gloves, a gown, a mask, and eye protection. After trimming is completed,the impression must be rinsed and disinfected. The knife or instrument used for trimming must also be disinfected.
Denture Repair Items
Denture repair items should always be disinfected in the “receiving” area, however, contamination can also occur when these items are being repaired. At a minimum, the areas and equipment used for denture repairs must be decontaminated at the end of every shift. Employees should take special care to remember to disinfect the pressure pot used for repairs (Figure 3)—it is a perfect incubator for bacterial growth because it is warm, moist, and dark.4
Shade Verification
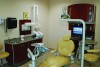
Shade verification and/or custom shade-taking is performed in some dental laboratories. Figure 4 shows the area at Drake Precision Dental Laboratory in Charlotte, NC where shade taking is typically performed. This procedure involves contact with the patient and as such, employers must assess how exposures could occur and train the workers who perform these tasks to protect themselves. Shade verification procedures should always involve the use of personal protective equipment and area decontamination should occur once the patient is dismissed.
Update the Exposure Control Plan Annually
Health and safety programs must undergo review annually (at a minimum) in order to remain up-to-date and address the most current health and safety concerns. As such, OSHA requires that Exposure Control Plans be reviewed at least once a year to avoid becoming outdated. Table 1 details how to execute an Exposure Control Plan review.
Use of Universal or Standard Precautions
When there is a risk of transmitting OPIM, employers must enforce the use of Universal or Standard Precautions. This entails treating all human blood and OPIM as if they are known to contain bloodborne pathogens. When practicing Standard Precautions, dental laboratory personnel would not treat items containing bloodborne pathogens any differently than others because they must treat all items that have been in a patient’s mouth as if they came from an infectious patient.
It is worth noting that dental offices should only release medical information about patients to those who have a need-to-know—and this does not necessarily apply to the dental laboratory. Sometimes, however, dental offices do share medical information with their laboratories (Figure 5). When this occurs, the laboratory must notify their clients that they have no need for these details, as they may pose a confidentiality risk.
There are a number of infectious diseases that dental patients could potentially pass on to dental laboratory employees. Additionally, many diseases—such as Hepatitis and HIV—can be asymptomatic and the patient may not know he or she is infected. Hepatitis B (HBV) is the foremost infection control concern in healthcare because it can survive outside of the body and still be capable of causing infection for at least 7 days. The CDC estimates that 800,000–1.4 million people in the United States have chronic HBV infections. Chronic infection is an even greater problem globally, affecting approximately 350 million individuals. An estimated 620,000 people worldwide die from HBV-related liver disease each year.5
All workers with occupational exposure to HBV must be trained on their risks of exposure and offered the HBV vaccine within 10 days of their initial assignment to a risk area. If a worker declines the vaccine, then he or she must sign a refusal form. OSHA provides the format for refusals on their website.6
Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States. Approximately 3.2 million persons are chronically infected.7 As far as Hepatitis B exposures in dentistry, the CDC has published information stating that there was an exposure in a dental health care setting in 2001 and in 2009 a cluster of 5 cases of acute Hepatitis B virus infections were reported among attendees of a two-day, portable dental clinic in West Virginia.8
HIV stands for human immunodeficiency virus and is the virus that can lead to acquired immunodeficiency syndrome, or AIDS. According to the CDC, unlike some other viruses, the human body cannot get rid of HIV. That means that once you have HIV, you have it for life.9 Today, an estimated 1.1 million people are living with HIV in the United States. People with HIV are now living longer than ever before because of treatments available. Anyone living with HIV needs to make choices that keep them healthy and protects others.10
Prevent, Correct, Control
Employers must prevent, correct, or control hazards once they are detected. OSHA recommends using the following to prevent and control hazards.11
Engineering Controls
Engineering controls are devices that isolate or remove a bloodborne pathogens hazard from the workplace. Engineering controls may include sharps disposal containers, safe devices for disposal of used needles in a dental office, hoods, and other exhaust in disinfecting areas.
Work Practice Controls
Work practice controls are practices that reduce the possibility of exposure by changing the way a task is performed. An example in a dental laboratory would be to use scissors, as shown in this photo, while trimming back over-extended borders on impressions instead of a utility knife to prevent cutting the hand (Figure 6).
Personal Protective Equipment
Personal protective equipment (PPE) is used when engineering controls and work practices do not provide adequate worker protection. The entry routes into the body must be determined and protected through the use of PPE such as gloves, gowns, eye protection, and masks.
Communicating Hazards
To protect workers and anyone else who may be handling regulated waste, employers must place warning labels on containers of regulated waste. Items that move between the dental office and the dentist that must be labeled with the biohazard emblem include extracted teeth, impressions, and implant components, among others (Figure 7). To prevent the disposal of biohazardous items in the dental laboratory, all packaging must be disinfected prior to disposal.
Provide Information and Training
Employers must train their workers on the risks of exposure upon initial assignment to a risk area and at least annually thereafter. The training must be presented at an educational level and in a language that workers understand. Workers must have the opportunity to ask the trainer questions and have those questions answered. Employers should always document this training and retain the records for a minimum of 3 years.
Post-Exposure Evaluation and Follow-Up
Even with all of these procedures in place, exposures can still happen. The Bloodborne Pathogens Standard requires employers to provide a post-exposure evaluation and follow-up to any occupationally-exposed worker who experiences an exposure incident. Examples of exposure incidents in a dental laboratory would be a cut that occurs when a worker is trimming back an over-extended border on an impression, or a stick with a sharp part of an appliance that has not been disinfected. When this type of exposure occurs, the employer must offer an evaluation at no cost to the worker. The dental laboratory must also notify the dentist, who must then contact the patient to request that he or she consent to blood testing. The employer must maintain confidentiality of the results of the testing for both the worker and the patient. Postexposure prophylaxis and counseling are also a part of this procedure. All exposure incidents must be documented, and the author recommends using the OSHA form available on their website.12
References
1. Bloodborne pathogens standard fact sheet. Occupational Safety and Health Administration Web site. https://www.osha.gov/OshDoc/data_BloodborneFacts/bbfact01.pdf. Accessed January 29, 2014.
2. Bloodborne pathogens. Occupational Safety and Health Administration Web site. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10051&p_table=STANDARDS. Accessed January 29, 2014.
3. Model exposure control plan. Occupational Safety and Health Administration Web site. https://www.osha.gov/OshDoc/Directive_pdf/CPL_2-2_69_APPD.pdf. Accessed January 29, 2014.
4. Bacterial growth. General Bacteriology Web site. http://generalbacteriology.weebly.com/bacterial-growth.html. Accessed January 29, 2014.
5. Hepatitis B FAQs for health professionals. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#overview. Accessed January 30, 2014.
6. Health care professionals Hepatitis B declination statement. Occupational Safety and Health Administration Web site. https://www.osha.gov/SLTC/etools/hospital/hazards/bbp/declination.html. Accessed January 29, 2014.
7. Hepatitis C information for health professionals. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HCV/index.htm. Accessed January 30, 2014.
8. Hepatitis B virus transmission in a dental office. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/OralHealth/infectioncontrol/factsheets/hepB.htm. Accessed January 27, 2014.
9. HIV basics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hiv/basics/index.html. Accessed January 29, 2014.
10. Living with HIV. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hiv/living/index.html. Accessed January 29, 2014.
11. Hazard prevention and control. Occupational Safety and Health Administration Web site. https://www.osha.gov/SLTC/etools/safetyhealth/comp3.html. Accessed January 29, 2014.
12. Bloodborne pathogens. Occupational Safety and Health Administration Web site. https://www.osha.gov/dte/grant_materials/fy09/sh-18796-09/bloodbornepathogens.pdf. Accessed January 29, 2014.
About the author
Mary A. Borg-Bartlett
President & Co-Founder
Safelink Consulting, Inc.
Cumming, GA