You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
In dentistry, it is often said, “You cannot diagnose what you cannot see”; in statistics, this concept is referred to as epistemic uncertainty. Epistemic uncertainty results from a lack of knowledge, and can occur when available information is incomplete or imprecisely evaluated. Improving knowledge acquisition is one way to avoid epistemic uncertainties. As part of this process, alternative explanations for a problem can be envisioned and experimental data can be gathered to support or refute them.
In dentistry, there is considerable epistemic uncertainty surrounding temporomandibular disorders (TMDs), especially the myofascial pain subtype. The hypothesis that dysfunctional sleep breathing may elicit temporomandibular joint (TMJ) pain will be examined as an alternative to traditional mechanical explanations, which focus on occlusion, malocclusion, and bruxism.
A previous article introduced the concept of sleep prosthodontics to differentiate it from sleep dentistry.1 Sleep prosthodontics is the study of the airway and its impact on the stomatognathic system, which encompasses the mouth, jaws, and the closely related structures of the oropharynx and fauces. Dentists become familiar with this system during its development, and assist in its maintenance throughout a patient’s life. This article will address the connection between sleep fragmentation and airway maintenance and the signs and symptoms of TMDs.
Prevalence and Symptoms
TMDs are a varied group of conditions that may affect the TMJ, the masticatory muscles, or both, and are the leading cause of nondental pain in the orofacial region. A classic presentation includes muscle or joint pain, joint sounds, and restricted or altered motion.2
In an attempt to create a standard for comparing findings from different TMD studies, the Research Diagnostic Criteria for Temporomandibular Disorders was established in 1992.3 Patients with known TMD are grouped by diagnostic criteria: muscle disorders (myofascial pain and myofascial pain with limited opening), disc displacements (with reduction, without reduction with limited opening, and without reduction without limited opening), or arthralgia/osteoarthritis/osteoarthrosis.2 In a systematic review, the prevalence of each group in TMD patients was as follows: myofascial pain, 45.3%; disc displacements, 41.1%; and arthralgia/osteoarthritis/osteoarthrosis, 30.1%.2 The most common of the subgroups was myofascial pain without limited opening and with more than three sites of pain on palpation.
As expected, prevalence of TMD was found to be lower in the general population, with 10.5% having myofascial pain subtype of TMD (M-TMD).2 Other studies have showed that the risk for TMDs is significantly elevated among women, especially black women.4 This article’s focus is limited to M-TMD, given that it is a common subtype of myofascial pain seen in patients presenting to restorative practices.
M-TMD is characterized by a dull, aching pain that worsens with palpation and function. Physical examination reveals hypersensitive regions or “trigger points” of taut skeletal muscle fiber. Cairns5 identified patients with M-TMD who complained of pain as exhibiting a localized hyperexcitability of the central nervous system when challenged with painful stimuli. The population seeking treatment for M-TMD comprises mostly women (3.8:1 ratio of women to men).6 The age of those diagnosed with M-TMD has been shown to range from 25 to 40 years, with prevalence decreasing with advancing age.7 This subtype of TMD has been shown to have variable pain intensity, as well as self-limiting and vacillating characteristics, making it difficult to create a framework for the natural progression of the disorder.8
A study was conducted by van Selms and colleagues9 to determine whether predictive factors for M-TMD pain could be identified over time. Patients were assessed before TMD treatment was initiated and were followed up for 12 months after resolution of pain symptoms. The pain complaints of patients with low baseline somatization scores continued to diminish over the 12-month period. Those with higher somatization scores at baseline had a measured relapse of complaints. It appears that somatization plays a role in the etiology and recurrence of M-TMD. This is interesting, given that many patients with TMD have functional somatic syndromes such as fibromyalgia, irritable bowel syndrome, and interstitial cystitis. These conditions are more associated with a generalized central nervous system hyperexcitability.10
Etiologic Theories for Myofascial Pain
Mechanical Theories
The mechanical explanations for myofascial pain that predominate the dental literature including occlusion, malocclusion, and bruxism. A prevailing etiologic theory is the connection between occlusion and TMD. The concept is that if the teeth are not in harmony with a centered condylar position in the fossa and/or if the guidance in function to a maximum intercuspal position has interferences, the muscle of mastication must reposition the mandible to allow proper function. This constant effort will create muscle dysfunction and M-TMD. Treatment options include occlusal adjustment, orthodontics, and orthognathic surgery.
Although 90% of the population does not have harmonious centric relation/maximum intercuspation positioning, and the majority have functional interferences, very few people have TMD.11 In addition, no systematic review has found evidence to indicate that occlusal adjustment leads to a greater degree of TMD resolution than placebo adjustment.12 Orthodontic interventions performed to improve occlusal relationships have also proved to not routinely resolve M-TMD and should not be considered a preventive measure or treatment option.13
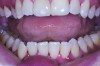
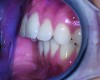
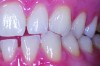
Although there are many malocclusions that do not lead to TMD symptoms, those such as unilateral cross bite, deep bite, increased overjet, and anterior open bite have been correlated with an increased risk of TMD.13 The underlying mechanism connecting these malocclusions with TMD may be the airway, given the bidirectional nature of the disorder. Many dentofacial physical risk indicators for malocclusion and TMD are also identified as indicators of increased risk for sleep-disordered breathing (Figure 1 through Figure 3). Measures aimed at prevention, reversal, and/or adequate treatment of malocclusion early in development might also help preclude negative health outcomes often associated with sleep−airway issues.
Another common mechanical theory is that force from bruxism is the pathogenic factor in myofascial pain. It seems that some patients also hold this belief. A recent study showed the self-reported rate of nighttime bruxism in patients with M-TMD (55.3%) to be higher than that of the general population (15.2%).14 However, polysomnography data failed to confirm this higher rate, and higher rates of bruxism were actually found in patients with less myofascial pain and in control subjects (Figure 4).
Biologic Theory: UARS
A biologic theory that serves as an alternative to the traditional mechanical theories is that hyperresponsive management of a more collapsible airway creates spontaneous pain and hyperalgesia. Within the fields of sleep medicine and sleep dentistry, recent focus has been placed on obstructive sleep apnea (OSA), which is characterized by complete upper airway obstruction lasting longer than 10 seconds with an associated 4% oxygen desaturation.1 It is most commonly attributed to a hypotonia of the soft palate or base of the tongue. Partial airway obstructions that lead to desaturation or brief awakenings from sleep are classified as hypopneas. Continued desaturation over time may cause excessive daytime sleepiness and medical comorbidities.
Upper airway resistance syndrome (UARS) was first described in the literature in 1993.15 Although many clinicians describe UARS and OSA as being the same disease with a slight variance in severity, their pathophysiologies in fact appear to differ.16 Anatomic irregularities or minor breathing impairments can cause UARS.17 Patients with UARS may have a more collapsible airway because of abnormal inspiratory flow dynamics18 or increased collapsibility on expiration due to atypical anatomy.19
Patients with UARS also differ from those with OSA in their responsiveness to the induction of an airway event. In patients with OSA, repetitive closures of the upper airway appear to dull the sensory receptors, causing an absence of activation of the dilator muscles in the airway.20 Therefore, patients with OSA may exhibit hyporesponsiveness or nonresponsiveness to upper airway collapse. Patients with UARS have more sensitivity to restricted breathing or negative oropharyngeal pressure, however, and airway constriction is recognized and responded to more quickly, preventing obstruction.
These respiratory effort–related arousals (RERAs) and sleep fragmentations lead to activation of the autonomic nervous system, particularly increased sympathetic nerve activity.21 This, in turn, causes a release of catecholamine (epinephrine and norepinephrine) into the bloodstream, creating a transient increase in pulse rate, blood pressure, and respiration. Catecholamine levels remain abnormally high for 24 hours after the event.22 This “fight-or-flight” response may be responsible for many of the damaging aspect of UARS.23
Polysomnography indicators for UARS include an apnea-hypopnea index score of 5 or less, five or more RERAs, and a minimum oxygen saturation level greater than 92%.24 Although some symptoms of UARS overlap with those of OSA, there are important distinctions between the two disorders. The typical patient with UARS is not overweight, for example. Women (typically aged 25-50 years) are three times more likely to be affected than men.24-26 It appears that women are more hyperresponsive to airway challenges because of the hormones progesterone and, to a slight degree, estrogen. Progesterone is a pharyngeal dilator and respiratory stimulator and also generates tongue muscle tone.27 Menstrual cycle phases alter hormone levels; one study found that more women complained of fatigue during the follicular phase, whereas there was a higher level of apnea (as measured by the apnea-hyponea index) in women during the luteal phase.28 The authors found that hormonal contraceptive use appeared to be associated with a reduced risk of airway dysfunction, improved sleep efficiency, and increased sleep duration.28 Most likely because of hormonal changes, postmenopausal women are more likely to snore and experience witnessed apneas, gasping, and frequent awakenings.29 In many cases, UARS develops into OSA in postmenopausal women.30
Chronic sleep-onset insomnia and sleep-maintenance insomnia are more common in patients with UARS.16 In a recent pilot study, 90% of the nocturnal awakenings experienced by patients with chronic insomnia were sleep breathing events, the majority of which were RERAs.31 All of the awakenings that lasted 5 minutes or longer, a duration that predisposes toward an insomnia episode, were preceded by a sleep-disordered breathing event.31 Adults with UARS may complain of fatigue rather than sleepiness.24 Higher incidence of sleepwalking, sleep terrors, myalgia, depression, and anxiety have been seen in patients with this disorder.25 Patients with UARS often present with a functional somatic syndrome misdiagnosis, including migraine/tension headaches, irritable bowel syndrome, chronic fatigue syndrome, TMD, and fibromyalgia.26 Many young women with sleep-disordered breathing are mistakenly treated with hypnotics, antidepressants, pain medication, attention-deficit/hyperactivity disorder medication, eugeroics, and muscle relaxants.32
The Link Between Pain and Sleep-Disordered Breathing
Sleep fragmentation may be culpable in cases of both allodynia, in which a normally inoffensive stimulus causes pain, and hyperalgesia, in which an increased response to a painful stimulus is experienced. Fragmented sleep profiles increase spontaneous pain or sensitize mechano-insensitive nociceptors with catecholamine, contributing to sympathetically maintained pain,33 and further impair natural pain-control mechanisms that are thought to play a key role in the development, maintenance, and exacerbation of chronic pain.34 Polysomnography reports on 25 consecutive patients who reported headache or palpable muscle pain revealed that 100% were diagnosed with UARS (J. Metz, personal communication, 2011).35
The phase of sleep that is reduced or eliminated by sleep fragmentation may also be important for maintaining homeostasis. Rapid eye movement (REM) sleep is a normal stage of sleep that usually accounts for 20% to 25% of total sleep time. It is considered the lightest level of sleep and is responsible for nondeclarative memory consolidation, anxiety/depression regulation, and pain control. Sleep deprivation, especially deprivation of REM sleep, induces spontaneous pain and hyperalgesia.35 During REM sleep, the only muscles that maintain normal tone are the diaphragm and those of the eyes. The remaining muscles are hypotonic, including the muscles that protect the airway. During the first extended REM sleep cycle of the evening, typically around 3 AM, the hyperresponsive patient with UARS cannot adequately protect his or her airway. Many patients wake with a large release of adrenaline and cannot return to sleep. In this case, a patient may lose the majority of REM sleep for the evening.
Women are more likely to experience sleep-disordered breathing during REM sleep (40.8%) than men (21.0%).36 As women age, the number of REM sleep disturbances they experience decreases, falling by 26.7% per decade; TMD proclivity data mimic REM sleep disturbance data.37 Postmenopausal women have been shown to experience more apnea and awakenings than premenopausal women.38
Inspiratory flow limitation with RERAs (ie, UARS) is associated with an increase in low-frequency sympathetic nervous system modulation.38 Apnea triggers awakenings but is associated with high-frequency parasympathetic activity, and does not create a sympathetic tone and pain response similar to UARS. Research that used cardiopulmonary coupling revealed that the ratio of damaging low-frequency to benign high-frequency spectral power was higher in the TMD group.39 Patients with TMD had greater sympathetic activation and patients with M-TMD were shown to have nocturnal heart rate variability and augmented sympathetic activity.39
Treatment
Although a full discussion of treatment protocols is beyond the scope of this article, the resolution of sleep-disordered breathing is critical because of all the negative effects it has on patient health and quality of life. Continuous positive airway pressure, mandibular advancement devices, neuromuscular onlays, and anterior repositioning splints have all been shown to be effective in the treatment of M-TMD, possibly due to the airway improvements they facilitate.40-43 Biofeedback and muscle exercises appear to be as effective.44,45
Traditional stabilization splint designs are routinely used in restorative dental practices. Despite their reparative advantages, they do not appear to be helpful in cases of sleep-disordered breathing. In fact, many patients with airway difficulties may experience an exacerbation of their condition when using a splint.46,47 Future studies are needed, but this may explain why many patients experience unsuccessful treatment or increased bruxing with their use, as well as why many remove the splint during the night.
Conclusion
A lack of knowledge, or the inaccurate interpretation of available data, can create epistemic uncertainty, a state of understanding that invites new approaches and additional research. The objective of this article was to explore sleep-disordered breathing as an alternative explanation for M-TMD, which had not been completely understood and addressed through current models in dentistry.
Data indicate that there is considerable overlap between patients with M-TMD and patients with UARS in terms of both demographics and signs and symptoms. Further research in the area of sleep prosthodontics represents an opportunity for innovative thinking. If dentistry begins to recognize that M-TMD could be a biologic problem rather than, or in addition to, a mechanical one, treatments could be designed to equilibrate to a systemic balance instead of an occlusal one.
References
1. Rouse JS. A new vision for dentistry. Inside Dentistry. 2013;9(7):60-78.
2. Manfredini D, Guarda-Nardini L, Winocur E, et al. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(4):453-462.
3. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301-355.
4. Janal MN, Raphael KG, Nayak S, Klausner J. Prevalence of myofascial temporomandibular disorder in US community women. J Oral Rehabil. 2008;35(11):801-809.
5. Cairns BE. Pathophysiology of TMD pain—basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil. 2010;37(6):391-410.
6. Michelotti A, Cioffi I, Festa P, et al. Oral parafunctions as risk factors for diagnostic TMD subgroups. J Oral Rehabil. 2010;37(3):157-162.
7. Machado LP, Nery Cde G, Leles CR, et al. The prevalence of clinical diagnostic groups in patients with temporomandibular disorders. Cranio. 2009;27(3):194-199.
8. deLeeuw R, ed. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. 4th ed. Chicago, IL: Quintessence Publishing Co; 2008:129-204.
9. van Selms MK, Lobbezoo F, Naeije M. Time courses of myofascial temporomandibular disorder complaints during a 12-month follow-up period. J Orofac Pain. 2009;23(4):345-352.
10. Balasubramaniam R, Laudenbach JM, Stoopler ET. Fibromyalgia: an update for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(5):589-602.
11. Reider C. The prevalence and magnitude of mandibular displacement in a survey population. J Prosthet Dent. 1978;39(3):324-329.
12. List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta-analyses. J Oral Rehabil. 2010;37(6):430-451.
13. Leite RA, Rodrigues JF, Sakima MT, Sakima T. Relationship between temporomandibular disorders and orthodontic treatment: a literature review. Dental Press J Orthod. 2013;18(1):150-157.
14. Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143(11):1223-1231.
15. Guilleminault C, Stoohs R, Clerk A, et al. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781-787.
16. Bao G, Guilleminault C. Upper airway resistance syndrome—one decade later. Curr Opin Pulm Med. 2004;10(6):461-467.
17. Chen W, Kushida CA. Nasal obstruction in sleep-disordered breathing. Otolaryngol Clin North Am. 2003;36(3):437-460.
18. Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27(3):459-466.
19. Woodson BT. Expiratory pharyngeal airway obstruction during sleep: a multiple element model. Laryngoscope. 2003;113(9):1450-1459.
20. Petrof BJ, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis. Sleep. 1996;19(6):465-471.
21. Walter LM, Foster AM, Patterson RR, et al. Cardiovascular variability during periodic leg movements in sleep in children. Sleep. 2009;32(8):1093-1099.
22. Zhang J, Ma RC, Kong AP, et al. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34(2):225-233.
23. Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185(1):177-185.
24. Exar EN, Collop NA. The upper airway resistance syndrome. Chest. 1999;115(4):1127-1139.
25. Guilleminault C, Kirisoglu C, da Rosa AC, et al. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7(2):163-170.
26. Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123(1):87-95.
27. Popovic, RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055-1062.
28. Hachul H, Andersen ML, Bittencourt L, et al. A population-based survey on the influence of the menstrual cycle and the use of hormonal contraceptives on sleep patterns in São Paulo, Brazil. Int J Gynaecol Obstet. 2013;120(2):137-140.
29. Tantrakul V, Park CS, Guilleminault C. Sleep-disordered breathing in premenopausal women: differences between younger (less than 30 years old) and older women. Sleep Med. 2012;13(6):656-662.
30. Jonczak L, Pływaczewski R, Sliwiński P, et al. Evolution of upper airway resistance syndrome. J Sleep Res. 2009;18(3):337-341.
31. Krakow B, Romero E, Ulibarri VA, Kikta S. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. Sleep. 2012;35(12):1685-1692.
32. Tantrakul V, Guilleminault C. Chronic sleep complaints in premenopausal women and their association with sleep-disordered breathing. Lung. 2009;187(2):82-92.
33. Jørum E, Ørstavik K, Schmidt R, et al. Catecholamine-induced excitation of nociceptors in sympathetically maintained pain. Pain. 2007;127(3):296-301.
34. Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494-505.
35. Roehrs T, Hyde M, Blaisdell B, et al. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145-151.
36. Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134(6):1156-1161.
37. Schwartz DJ, Moxley P, Barker A, Longman M. On a characteristic of cortical arousals in individuals with obstructive sleep apnea. J Clin Sleep Med. 2005;1(1):35-40.
38. Chandra S, Sica AL, Wang J, et al. Respiratory effort-related arousals contribute to sympathetic modulation of heart rate variability [published online ahead of print February 18, 2013]. Sleep Breath.
39. Eze-Nliam CM, Quartana PJ, Quain AM, Smith MT. Nocturnal heart rate variability is lower in temporomandibular disorder patients than in healthy, pain-free individuals. J Orofac Pain. 2011;25(3):232-239.
40. Goksan B, Gunduz A, Karadeniz D, et al. Morning headache in sleep apnoea: clinical and polysomnographic evaluation and response to nasal continuous positive airway pressure. Cephalalgia. 2009;29(6):635-641.
41. Giannasi LC, Almeida FR, Magini M, et al. Systematic assessment of the impact of oral appliance therapy on the temporomandibular joint during treatment of obstructive sleep apnea: long-term evaluation. Sleep Breath. 2009;13(4):375-381.
42. Cooper BC, Kleinberg I. Establishment of a temporomandibular physiological state with neuromuscular orthosis treatment affects reduction of TMD symptoms in 313 patients. Cranio. 2008;26(2):104-117.
43. Brown DT, Gaudet EL Jr. Temporomandibular disorder treatment outcomes: second report of a large-scale prospective clinical study. Cranio. 2002;20(4):244-253.
44. Niemelä K, Korpela M, Raustia A, et al. Efficacy of stabilisation splint treatment on temporomandibular disorders. J Oral Rehabil. 2012;39(11):799-804.
45. Shedden Mora MC, Weber D, Neff A, Rief W. Biofeedback-based cognitive-behavioral treatment compared with occlusal splint for temporomandibular disorder: a randomized controlled trial [published online ahead of print February 26, 2013]. Clin J Pain.
46. Nikolopoulou M, Naeije M, Aarab G, et al. The effect of raising the bite without mandibular protrusion on obstructive sleep apnoea. J Oral Rehabil. 2011;38(9):643-647.
47. Gagnon Y, Mayer P, Morisson F, et al. Aggravation of respiratory disturbances by the use of an occlusal splint in apneic patients: a pilot study. Int J Prosthodont. 2004;17(4):447-453.
About the author
Jeffrey S. Rouse, DDS
Private Practice
San Antonio, Texas